3445
A rapid quantitative Multi-inversion SAGE-EPI brain protocol with subspace reconstruction and navigation-free shot-to-shot phase correction1A.A. Martinos Center for Biomedical Imaging, Boston, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Department of Electrical Engineering and Computer Science, Massachusetts Institution of Technology, Cambridge, MA, United States, 4Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States
Synopsis
Recently, multi-contrast EPI approaches have been proposed for a fast screening brain protocol. With EPI-encoding, multiple contrasts can be acquired quickly for robust, quantitative mapping as well as creation of synthetic weighted images. Here, a spatiotemporal subspace reconstruction is developed to jointly reconstruct multi-contrast multishot-EPI data from a multi-inversion Spin and Gradient echo EPI (MI-SAGE-EPI) acquisition. An approach to estimate and incorporate shot-to-shot phase corruption into the reconstruction was also developed. This navigation-free subspace reconstruction achieves good reconstruction for MI-SAGE-EPI at a high EPI-acceleration, thus enabling a rapid quantitative protocol at high in-plane resolution with minimal distortion and blurring.
Introduction
Current clinical brain imaging protocols are typically on the order of 15 minutes or more and involve several separate scans to achieve all desired contrasts. Recent studies have investigated using efficient sampling to acquire many desired contrasts in one fast protocol1–3. In particular, with EPI-encoding, multiple contrasts can be acquired quickly for robust, quantitative mapping as well as creation of synthetic weighted images. Nonetheless, the ability of EPI to provide rapid imaging comes at the cost of detrimental image distortion and blurring in the phase encoding direction due to B0 inhomogeneities and T2* decay, particularly at high resolution. One method to reduce these problems is to use multi-shot EPI (msEPI), however, this can create image artifacts due to shot-to-shot phase variations, for example from B0-variations from breathing. Here a spatiotemporal subspace reconstruction4–7 is developed to jointly reconstruct a large number of multi-contrast msEPI data from a multi-inversion Spin and Gradient Echo EPI (MI-SAGE-EPI) acquisition. An approach to estimate and incorporate shot-to-shot phase corruption into this reconstruction was also developed. This navigation-free subspace reconstruction was demonstrated to achieve good reconstruction with high-SNR for MI-SAGE-EPI at a high acceleration, thus enabling a rapid quantitative protocol with high in-plane resolution and minimal distortion and blurring.Methods
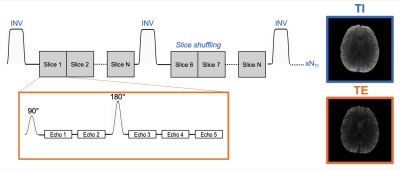
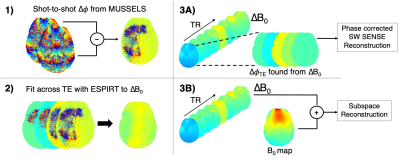
The MI-SAGE-EPI sequence3, that combines multi-inversion8,9 and multi-echo10 EPI concepts, was used to achieve PD, T1, T2, and T2* weighting in a single sequence rapidly across multiple slices (Figure 1). The following sequence parameters were used: FOV = 220x220 mm, 20 slices, resolution = 1x1x5 mm, TE = [18 45 73 100 127] ms, TR = 4.2s, 8 inversion acquisitions with slice-order-shuffling, total contrasts per slice = TI x TE = 8 x 5 = 40, Rinplane = 8, with 2 EPI-shots (total scan time 67 sec + 20 sec reference scan).Shot-to-shot phase estimation: An important ingredient for achieving high-quality joint reconstruction of multi-contrast msEPI data with spatiotemporal subspace reconstruction is a robust approach to estimate and account for shot-to-shot phase errors. The structured low-rank matrix completion11 approach termed MUSSELS12 has had good success in estimating and correcting phase errors in ms-EPI. However, at high accelerations, this reconstruction approach still resulted in significant artifacts. An improved phase estimate approach developed in this work builds upon and refines the phase estimate from MUSSELS as outlined in Figure 2. Here, the phase differences ($$$\Delta\Phi$$$) found from an initial MUSSELS reconstruction of msEPI data for each individual contrast (Rinplane=8, 2-shots) were used as an initial estimate. An ESPIRIT13 like fitting-model was then applied to smooth/refine $$$\Delta\Phi$$$ maps across echoes (treating TEs as coils), and a common B0 difference map ($$$\Delta$$$B0) was fit across echoes of each SAGE readout to further improve the estimate. This common $$$\Delta$$$B0 map could then be extrapolated out to create $$$\Delta\Phi$$$ for all echoes, for subsequent reconstruction as needed.
The shot-to-shot phase estimates are incorporated into a spatiotemporal subspace4,6 reconstruction where the shot specific $$$\Delta$$$B0 is added to a background phase B0 estimate (found from a multi-echo gradient echo reference scan, >20 sec) and included in the forward model. The subspace reconstruction simulated the signal evolution within a range of expected T1, T2, and T2* values, and applied a singular value decomposition to find a low-dimensional number of bases to represent the signal evolution across all of the possible parameter space. This was incorporated into the SENSE model directly to reduce image noise and improve reconstruction performance.
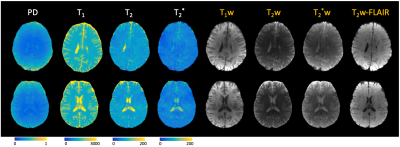
After reconstruction, the images were fit voxel-wise using a dictionary matching approach derived from Bloch simulations to quantitative PD, T1, T2, and T2* maps. The quantitative maps were also used to generate synthetic weighted images (T1w-, T2-w, T2*-w, and T2w-FLAIR) using typical sequence parameters.
Results
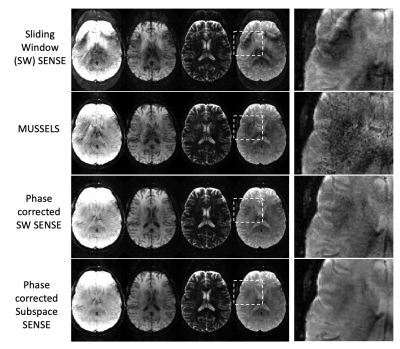
Figure 3 shows selected contrasts from one slice comparing results from a standard sliding-window SENSE reconstruction (across EPI-shots), MUSSELS reconstruction, sliding-window SENSE with the shot-specific phase estimate incorporated, and a subspace reconstruction with 16 basis functions. Image artifacts are largely reduced using the shot-to-shot phase estimation, and image quality and SNR are further improved using the subspace reconstruction. Figure 4 shows quantitative maps and synthetic weighted images from two representative slices from different subjects that were generated from the subspace reconstruction.Discussion
These results show the benefit of a subspace reconstruction with a phase correction term to allow for high in-plane acceleration, to minimize echo times, distortion, and blurring, by enabling a multi-shot acquisition without image corruption from phase differences across shots. These corrections allowed for a 1mm in plane EPI acquisition with minimal distortion to achieve quantitative PD, T1, T2, and T2* maps in just over a minute. Future work includes refinement to the $$$\Delta$$$B0 estimate to further improve reconstruction and allow for higher accelerations. Additionally, the acquired echo times may be further optimized, by shuffling or shifting acquired TEs at different inversion times, to improve complementary sampling for the joint subspace reconstruction and increase performance at high accelerations.Acknowledgements
This work was supported in part by NIH research grants: F32EB026304, R01MH116173, R01EB020613, R01EB019437, U01EB025162, P41EB015896, and the shared instrumentation grants: S10RR023401, S10RR019307, S10RR019254, S10RR023043References
1. Tanenbaum LN, Tsiouris AJ, Johnson AN, et al. Synthetic MRI for Clinical Neuroimaging: Results of the Magnetic Resonance Image Compilation (MAGiC) Prospective, Multicenter, Multireader Trial. Am J Neuroradiol. 2017;38(6):1103-1110. doi:10.3174/ajnr.A5227
2. Skare S, Sprenger T, Norbeck O, et al. A 1-minute full brain MR exam using a multicontrast EPI sequence. Magn Reson Med. 2018;79(6):3045-3054. doi:10.1002/mrm.26974
3. Manhard MK, Liao C, Stockmann J, et al. Combined T1 , T2 , and T2 * mapping using a multi-inversion multi-echo spin and gradient echo EPI sequence. Proc 27th Annu Meet ISMRM, Montr Canada. 2019. doi:10.1002/mrm.26382.2.
4. Liang Z-P. Spatiotemporal imaging with partially separable functions. In: 2007 4th IEEE International Symposium on Biomedical Imaging: From Nano to Macro. IEEE; 2007:988-991. doi:10.1109/ISBI.2007.357020
5. Tamir JI, Uecker M, Chen W, et al. T2 shuffling: Sharp, multicontrast, volumetric fast spin-echo imaging. Magn Reson Med. 2017;77(1):180-195. doi:10.1002/mrm.26102
6. Dong Z, Wang F, Reese TG, Bilgic B, Setsompop K. Echo Planar Time-Resolved Imaging (EPTI) with subspace constraint and optimized kt trajectory. Proc 27th Annu Meet ISMRM, Montr Canada. 2019:2-4.
7. Zhao B, Lu W, Hitchens TK, Lam F, Ho C, Liang Z-P. Accelerated MR parameter mapping with low-rank and sparsity constraints. Magn Reson Med. 2015;74(2):489-498. doi:10.1002/mrm.25421
8. Clare S, Jezzard P. RapidT1 mapping using multislice echo planar imaging. Magn Reson Med. 2001;45(4):630-634. doi:10.1002/mrm.1085
9. Renvall V, Witzel T, Wald LL, Polimeni JR. Automatic cortical surface reconstruction of high-resolution T1echo planar imaging data. Neuroimage. 2016;134:338-354. doi:10.1016/j.neuroimage.2016.04.004
10. Schmiedeskamp H, Straka M, Newbould RD, et al. Combined spin- and gradient-echo perfusion-weighted imaging. Magn Reson Med. 2012;68(1):30-40. doi:10.1002/mrm.23195
11. Haldar JP. Low-Rank Modeling of Local k-Space Neighborhoods (LORAKS) for Constrained MRI. IEEE Trans Med Imaging. 2014;33(3):668-681. doi:10.1109/TMI.2013.2293974
12. Mani M, Jacob M, Kelley D, Magnotta V. Multi-shot sensitivity-encoded diffusion data recovery using structured low-rank matrix completion (MUSSELS). Magn Reson Med. 2017;78(2):494-507. doi:10.1002/mrm.26382
13. Uecker M, Lai P, Murphy MJ, et al. ESPIRiT - An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med. 2014;71(3):990-1001. doi:10.1002/mrm.24751
Figures