3422
Quantitative Assessment of Metal Artefact Reduction Sequence Used In Imaging in Patients with Orthopaedic Total Hip Replacement Implants1Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom, 2King's College London, London, United Kingdom, 3Johns Hopkins University, Baltimore, MD, United States
Synopsis
Musculoskeletal (MSK) imaging of patients with hip implants can be challenging due to metal artefacts. We have quantitatively assessed a number of metal artefact reduction sequences (MARS) on commonly implanted hip implants. The MARS methods used were: high receiver bandwidth, high transmit bandwidth, view angle titling (VAT) and slice encoded metal artefact correction (SEMAC). Signal pile-up and voids were most reduced across implants when SEMAC was employed and also artefacts at the bone metal interface were reduced.
Purpose
Musculoskeletal (MSK) imaging of patients with hip implants can be challenging due to metal artefacts. The metallic composition of hip implants varies between models, resulting in a range in the level of metal artefact from virtually none to images that are completely non-diagnostic. Most clinical MR systems have a range of metal artefact reduction sequences (MARS) to counteract these effects [1]. The aim of this study was to quantify artefact reduction capabilities of various metal artefact reduction MRI pulse sequences in 10 commonly used total hip replacement implants (THRIs) used worldwide.Implants
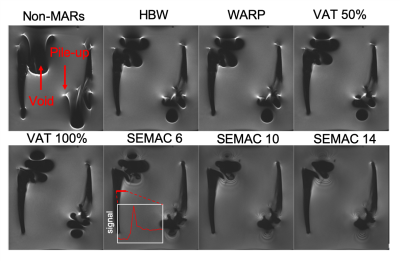
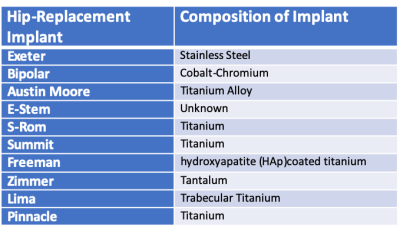
10 THRIs were examined and are shown in Figure 1. Table 1 shows the metallic composition of each implant.Methods
MRI Acquisition10 THRIs were imaged on a Siemens Aera 1.5 T MRI system in a water-based phantom. T1-weighted images were acquired with & without applying the following metal artefact reduction sequences (MARS):
- high receiver bandwidth
- high transmit bandwidth (WARP)
- view-angle-titling (VAT) at 50% and 100% [2]
- slice encoded metal artefact correction (SEMAC) with 6, 10 and 14 encoding steps. [3] (VAT is set to 100% by default when SEMAC is used)
Artefact Analysis
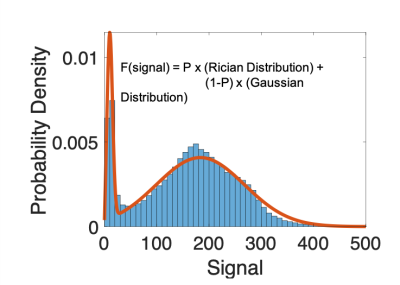
The size of signal voids and pile-up were assessed via histogram analysis of the signal distribution to the signal around each implant (Figure 2). One ROI was drawn for each implant that was large enough to cover both signal void and pile-up. The MR signal surrounding the implant not effected by the metal artefact was modelled as a Gaussian distribution and the signal measured within the void as a Rician distribution (which is used to model noise):
$$ Signal = p (Rician Distribution) + (1-p) (Gaussian Distribution) $$.
Where p represents the probability that the MR signal is within the Rician distribution. The size of the ROI remained the same across MARS images to allow assessment of the effects of the MARS techniques. The change in probability from the non-MARS image that the signal fell under the Rician was used to assess the reduction in the signal void. Signal pile-up around the boundaries of the implant was measured by assessing the number of voxels that fell outside 3 standard deviation above the mean.
Additionally, line profiles were drawn across the stem of the implants to assess signal pile-up at the bone-metal interfaces.
Results
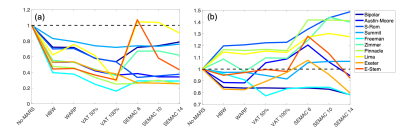
Signal pile-up (the number of voxels greater than 3 standard deviations from mean of the fitted Gaussian) was reduced for all MARS techniques, shown in Figure 4a, in all implants with SEMAC (factor=14) and VAT (100%) both performing the best, with a reduction of pile-up between 28% and 75%. The E-Stem caused large a signal void, therefore using a small SEMAC factor of 6 - it struggled to correct for the distortion.MARS reduced the signal void in 7/10 THRIs – shown in Figure 4b, with SEMAC (factor=14) outperforming all other methods and, the signal void reduced in the 7 THRIs ranging between 6% to 22%.
At the bone-metal interface of all 7 hip implants which had femoral stems, SEMAC (factor=14) reduced signal pile-up by a range of 11% to 86%.
Conclusion
Implant design and metallurgy dependant, the signal void could be reduced up to 22% using SEMAC. All MARS methods reduced the signal pile-up with SEMAC and VAT performing the best. Whilst SEMAC performed well, there are large variation in performance of MARS methods between implants. In a clinical environment there is a lack of a priori knowledge in regards to the make and model of the implant. Thus, making it difficult to judge which MARS to use.SEMAC incurs a significant time-penalty which may be problematic in certain scenarios, for example patients that cannot remain still for a significant amount of time. SEMAC significantly reduces signal pile-up at bone-metal interface, improving the diagnostic ability of MRI for periprosthetic complications and pathologies including tumour recurrence, collections, fractures & muscle evaluations as well as AI-guided preoperative revision surgery planning.
It is possible to modify the SEMAC based sequences such that there can be a reduction the scanning time, which include a trade-off between voxel sizes, slice thickness and parallel imaging acceleration. A more robust strategy would be to utilise compressed sensing [4] however this currently incurs additional cost implications which restrict its availability to a wide cohort of patients.
Acknowledgements
No acknowledgement found.References
[1] Hargreaves, Brian A., et al. "Metal-induced artifacts in MRI." American Journal of Roentgenology 197.3 (2011): 547-555.
[2] Butts, Kim, John M. Pauly, and Garry E. Gold. "Reduction of blurring in view angle tilting MRI." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 53.2 (2005): 418-424.
[3] Lu, Wenmiao, et al. "SEMAC: slice encoding for metal artifact correction in MRI." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 62.1 (2009): 66-76.
[4] Fritz, Jan, et al. "Compressed sensing SEMAC: 8-fold accelerated high resolution metal artifact reduction MRI of cobalt-chromium knee arthroplasty implants." Investigative radiology 51.10 (2016): 666-676.
Figures