3417
Diffusion spoiling for fast steady-state gradient echo imaging of the human brain using a 300 mT/m gradient system1Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
Synopsis
In gradient echo imaging sequences, transverse coherences persisting across repetitions must be managed to avoid large variations in the steady state signal. Ultra-high amplitudes for gradient spoiling can be effective, however, conventional human MR scanners do not achieve the necessary gradient amplitudes. By using a 3T Connectom MRI scanner with a 300 mT/m gradient amplitude, we demonstrate that by operating in the diffusion spoiling regime, it is now possible to eliminate the contribution of unwanted transverse magnetization to the steady state by gradient spoiling alone. It can serve as a reference standard for validating spoiling schemes and for R1 quantification.
Introduction
In a gradient echo imaging sequence with repetition times (TR) close to or shorter than the transverse relaxation time (T2), transverse coherences persisting across repetitions must be managed to avoid large variations in the steady state signal. Such variations may result in visible image artifacts, or affect the accuracy of quantitative techniques such as multiparameter mapping1-3.To achieve T1-weighting, commonly a mix of gradient4 and RF5 spoiling is applied to achieve a steady state in the longitudinal magnetization and minimal transverse coherences at the end of each TR. Using ultra-high amplitudes for the gradient spoilers can achieve a spoiling regime where transverse coherences are suppressed by diffusion effects, the so-called strong gradient spoiling regime. However, conventional human MR scanners do not achieve the necessary gradient amplitudes and thus only weak and intermediate gradient spoiling regimes are possible, meaning a significant dependence of the measured signal on the quadratically-applied RF phase increment remains, leading to instabilities and bias in quantitative imaging2. In this work, we used a 3T Connectom MRI scanner (Siemens Healthineers, Erlangen, Germany) with a 300 mT/m gradient amplitude to demonstrate that by operating in the diffusion spoiling regime, it is now possible to eliminate the contribution of unwanted transverse magnetization to the steady state by gradient spoiling alone for human neuroimaging applications.
Methods
In vivo data were acquired on a 3T Connectom MR system (max. gradient strength 300 mT/m), using a 3D multi-echo gradient echo sequence with RF and gradient spoiling flexibly configurable. By varying the amplitude of spoiler gradient lobes applied on the read axis by up to 290 mT/m, we achieved spoiling moments ranging from 70.5 (mT/m)ms (equivalent to the 6π/pixel dephasing typically applied in such protocols) to 1000 (mT/m)ms.Other parameters followed standard neuroimaging protocols: 1 mm isotropic resolution, 8 echoes, TE equally distributed between 2.4 and 18.5 ms, TR of 26 ms, and GRAPPA factor 2 applied in both phase and partition encoding directions for an imaging time of 4 min 55 s.
For each diffusion condition volumes with PD- and T1-weighting (flip angles 6°, 21° respectively) were acquired, along with reference field maps for B1+ correction6. MATLAB, SPM12 and the hMRI toolbox7 were used to calculate quantitative maps of R1. No post hoc corrections for imperfect spoiling of transverse coherences were applied3,7. To further demonstrate the effectiveness of the diffusion spoiling, additional images were acquired without RF spoiling but with 70.5 and 1000 (mT/m)ms spoiler moments.
Results and discussion
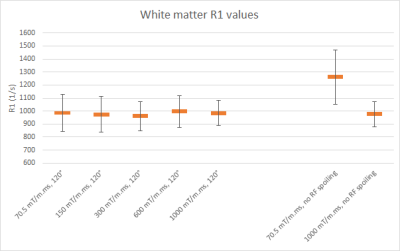
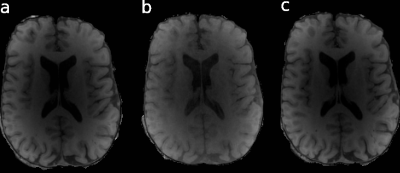
In vivo estimations of R1 showed little influence on the spoiling moment applied (Figure 1) with phase increment ɸ = 120°. In the weak spoiling regime, R1 in white matter is ca. 30% higher when RF spoiling is disabled. In the diffusion spoiling regime, the dependence on the RF phase increment appears absent, though future work could provide confirmation with a more exhaustive search over phase increment values.Fig. 2 shows that even without any RF spoiling, the diffusion spoiling suppresses effectively the transverse coherences and achieves T1-weighting with clear gray/white matter contrast (Fig. 2c) similar to RF spoiled acquisitions (Fig. 2a), unlike the weak/intermediate spoiling regime acquisition without RF spoiling (Fig. 2b).
Conclusion
With a high performance gradient system, spoiling moments required for the diffusion based strong spoiling regime can be achieved in otherwise standard steady state gradient echo acquisitions without lengthy extensions of scan time. Since the spoiling efficiency in this regime is largely independent of the subtleties of RF spoiling, gradient spoiling and even excitation flip angle, it offers new opportunities. For example, it can serve as a reference standard for validating spoiling schemes and provide references for R1 quantification.Acknowledgements
This project has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 616905. NW has received funding from the BMBF (01EW1711A & B) in the framework of ERA-NET NEURON. The authors thank Antoine Lutti for sequence development.References
1. Weiskopf N, Suckling J, Williams G, et al. Quantitative multi-parameter mapping of R1, PD*, MT, and R2* at 3T: a multi-center validation. Front Neurosci. 2013;7:95.
2. Yarnykh VL. Optimal radiofrequency and gradient spoiling for improved accuracy of T1 and B1 measurements using fast steady-state techniques. Magn Reson Med. 2010 Jun;63(6):1610-26.
3. Preibisch C and Deichmann R. Influence of RF spoiling on the stability and accuracy of T1 mapping based on spoiled FLASH with varying flip angles. Magn Reson Med. 2009 Jan;61(1):125-35.
4. Crawley AP, Wood M and Henkelman RM. Elimination of transverse coherences in FLASH MRI. Magn Reson Med. 1988 Nov;8(3):248-260.
5. Zur Y, Wood ML and Neuringer LJ. Spoiling of transverse magnetization in steady-state sequences. Magn Reson Med. 1991 Oct;21(2):251-63.
6. Lutti A, Hutton C, Finsterbusch J, et al. Optimization and Validation of Methods for Mapping of the Radiofrequency Transmit Field at 3T. Magn Reson Med. 2010 Jul; 64(1): 229-238.
7. Tabelow K, Balteau E, Ashburner J, et al. hMRI - A toolbox for quantitative MRI in neuroscience and clinical research. Neuroimage. 2019 Jul 1;194:191-210.
Figures