3411
Imaging the Internal Acoustic Meatus (IAMs) of patients with cochlear implants in-situ using Slice Encoded Metal Artefact Reduction.1Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom
Synopsis
Neurofibromatosis type 2 (NF2) patients commonly have bilateral vestibular schwannomas, which typically require regular monitoring with MRI. However these patients also have cochlear implants which makes MRI challenging. We have used Slice Encoded Metal Artefact Correction (SEMAC) to improve the imaging of IAMs in patients with cochlear implants in-situ. The implementation of SEMAC imaging reduced the geometric distortion and the volume of the signal void around the implants by over half.
Purpose
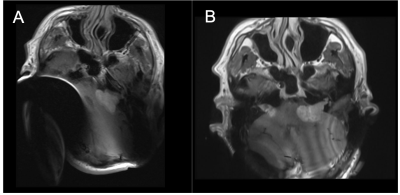
Neurofibromatosis type 2 (NF2) is a genetic condition that results in the growth of tumours in the nervous system [1]. It is commonly associated with bilateral vestibular schwannomas, which typically require regular monitoring with MRI.Many NF2 patients develop significant hearing loss and go on to have cochlear implants. Although many cochlear implants are MR Conditional for scanning at 1.5T, the magnet that is typically associated with the implant results in a significant artefact around the implant, making MRI challenging. This is demonstrated in Figure 1a where the bilateral vestibular schwannomas are obscured. One route to improve imaging is to remove the magnets prior to the MRI. This however requires surgery, which is time consuming, is costly and not without its own risks to the patient.
Slice Encoded Metal Artefact Correction (SEMAC) [2] is a technique for reducing metal artefacts. The downside of SEMAC is an acquisition time penalty, so there is trade-off to be made between reduced metal artefact (from an increased SEMAC factor) and acquisition time.
The aims of this work were firstly to investigate the relationship between artefact size and SEMAC factor, and secondly to evaluate the impact of SEMAC on visualisation of IAMS in an NF2 patient population.
Methods
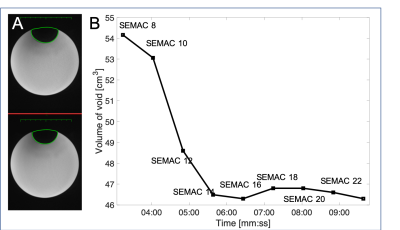
An Octicon Medical Neuro ZtiEVO cochlear implant was attached to a cylindrical doped water phantom and imaged using a 1.5T Siemens Aera. Axial T1w images were acquired over a range of SEMAC factors: 8 to 24. [FOV=200x162mm2, matrix size=312x512, bandwidth=512Hz/px, VAT factor=100%, slices=20, flip angle=90, GRAPPA=2]. The volume of the void was measured across all slices by drawing an ROI on each affected slice.Eight patients with cochlear implants in-situ (one of which had bilateral implants) were scanned on a 1.5T Siemens Aera. Axial T1w images [FOV=200x162mm2, matrix size=312x512, bandwidth=512Hz/px, VAT factor=100%, slices=20, flip angle=90, GRAPPA=2, scan-time=6.30mins] through the IAMs were acquired pre-contrast without SEMAC and post-contrast with a SEMAC factor of 16. The volume of the void for both sets of images was measured.
Quantitative scoring of all clinical images was performed by a consultant radiologist. They scored the quality of the appearance of the IAMs and the cerebellum using the following scale: 1=unusuable/completely obscured, 2=inadequate/minority structures seen/moderate significant distortion, 3 good=majority structures seen/mild distortion, 4=no obscuration or distortion.
Results
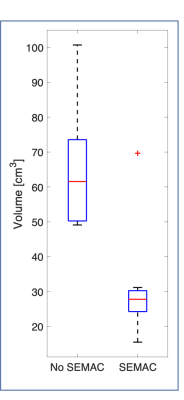
Figure 2 shows the results from the phantom analysis. Increasing the SEMAC factor reduced the volume of the void surrounding the implant, with a minimum achieved of 46cm3 at SEMAC factor between 14 and 16.Figure 3 shows the results of the patient study. The signal void reduced in all cases, on average from 61cm3 to 28cm3.
In 83% of the cases where SEMAC was not used the image quality was either unusable or inadequate, compared to just 16% when SEMAC was employed. In 91% of cases the cerebellum was unusable or inadequate when SEMAC was not used compared to just 8% of cases when SEMAC was employed.
Discussion and Conclusion
This work demonstrates that SEMAC can be successfully employed to improve the imaging of IAMs in patients with cochlear implants in-situ. The implementation of SEMAC imaging reduced the geometric distortion and the volume of the signal void around the implants by over half. The improvements were significant in that they allowed the radiologist to better visualise and measure both the bilateral vestibular schwannomas. These patients typically have follow-up imaging every 3/6 months and therefore accurate and repeatable imaging is important in the patient treatment pathway.However, there are still several hurdles to be overcome. Significant signal voids still remain despite SEMAC, and the gains in signal come at the cost of increased scan durations.
Future work will explore further optimisations to the SEMAC acquisition. This will include increasing the FOV in the z-direction for each SEMAC volume and exploring what slice frequency offsets will allow maximum signal recovery in the vicinity of the implant. We will also explore how to optimise CI placement to minimise signal loss impingement on the IAM.
Acknowledgements
Authors would like to thank Octicon Medical for providing a Neuro ZtiEVO cochlear implant.References
[1]Evans, D Gareth R. "Neurofibromatosis type 2 (NF2): a clinical and molecular review." Orphanet journal of rare diseases 4.1 (2009): 16.
[2] Lu, Wenmiao, et al. "SEMAC: slice encoding for metal artefact correction in MRI." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 62.1 (2009): 66-76.
Figures