3408
Interleaved reversed polarity MPRAGE for in vivo B0 readout distortion correction1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Wellcome Centre for Integrative Neuroimaging, University of Oxford, Oxford, United Kingdom, 4Computer Science and Artificial Intelligence Laboratory, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Readout distortion can affect structural imaging with MPRAGE particularly in temporal regions. Interleaved readout polarity MPRAGE allows for estimation of distortion without motion confound between oppositely distorted images. This approach could be useful for single echo MPRAGE imaging with ~200 Hz/px.
Introduction
In vivo MPRAGE can suffer from B0 distortions in the readout direction, particularly for long readout gradients that have low bandwidth per pixel (~200 Hz/px). Multi-echo MPRAGE reduces the distortion by acquiring 4 shorter echoes with higher readout bandwidth per pixel (~650 Hz/px) that are typically root-mean-square (RMS) combined to recover SNR1. In ex vivo ultra-high resoltution imaging, readout gradients can be even longer with bandwidth per pixel ~100 Hz/px, and we have recently shown that reversed readout gradient acquisitions can be used to correct distortion2 using the TOPUP tool developed for spin-echo EPI images with reversed phase-encoding (blip up/down)3. Recently, EPI has been modified to acquire interleaved blip up/down data for dynamic estimation and correction of B0 distortion in functional MRI4.In this study, we implemented interleaved readout (RO) polarity MPRAGE that acquires each TR (ky phase-encoding) with opposite readout polarity to provide two registered images with opposite readout distortion. These were used to assess and correct distortion both in single- and multi-echo 1 mm isotropic MPRAGE at 3 and 7T.
Methods
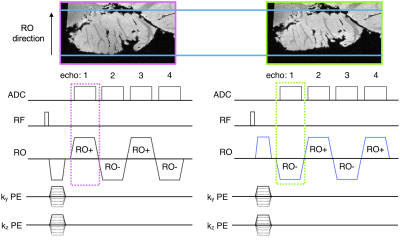
3D MPRAGE was modified to acquire interleaved opposite RO polarities in successive TRs, as shown in Fig. 1, and to reconstruct the separate images on the scanner.Two healthy volunteers were scanned in accordance with institutional review board guidelines. Single- and multi-echo data were acquired at 3T with 1 mm isotropic resolution, FOV 256x240x176 mm, water excitation for fat signal suppression, flip angle 7 degrees. At 3T, 8 mm isotropic resolution vNavs5 were embedded in the MPRAGE scans to assess motion during interleaved RO scans and separate reversed RO scans (data not shown).
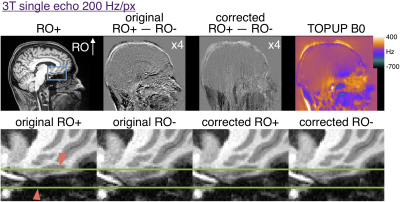
3T single echo MPRAGE parameters: 200 Hz/px; echo-spacing=8.9ms; TR/TI=2500/1140ms; TE=3.3ms; R=3 GRAPPA acceleration with 32 integrated reference lines; scan time=8:42 min.
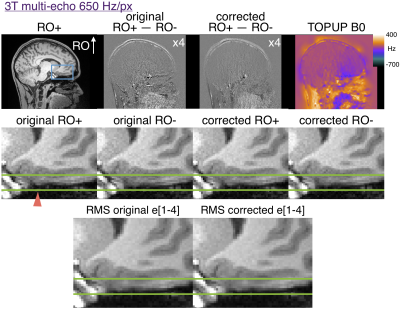
3T multi-echo MPRAGE parameters: 650 Hz/px; echo-spacing=11.1ms; TR/TI=2500/1330ms; TE=[1.69, 3.55, 5.41, 7.27]ms; R=2 GRAPPA acceleration with 32 integrated reference lines; scan time=11:37 min. 7T data were acquired with 1 mm isotropic resolution, FOV 256x256x176 mm, water excitation, FOCI refocusing pulse6, flip angle 7 degrees.
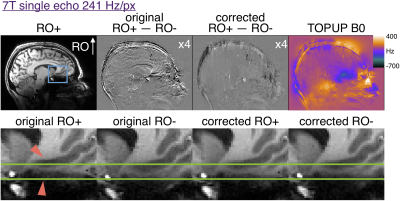
7T single echo MPRAGE parameters: 241 Hz/px; echo-spacing=7.5ms; TR/TI=2500/1100ms; TE=3.3ms; R=3 GRAPPA acceleration; scan time=8:52 min
7T multi-echo MPRAGE parameters: 651 Hz/px; echo-spacing=10.4ms; TR/TI=2500/1100ms; TE=[2.12, 3.98, 5.84, 7.7]ms; R=2 GRAPPA acceleration; scan time=8:52 min.
In multi-echo scans the first echoes with opposite RO polarity were used to estimate a distortion field with TOPUP as implemented in FSL3,7. The subsequent echoes were corrected and RMS-combined. Similarly, the opposite RO single echo images were used to estimate distortion before both polarities were corrected for comparison. Difference maps of the RO+ and RO- first echo were generated before and after distortion correction.
Results
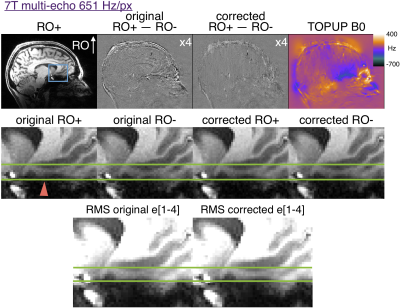
Figure 2 shows that 200 Hz/px MPRAGE has substantial distortion at 3T and that the opposite polarity images can be aligned with TOPUP. The original RO+ and RO- are shifted by ~2 pixels in regions of ~400Hz off resonance. Figure 3 shows that multi-echo MPRAGE with 650 Hz/px bandwidth substantially reduces distortion and there is no perceptible difference in the final RMS images.Figure 4 shows the increased level of distortion at 7T with more edges visible in the original difference map. TOPUP correction can successfully align the RO+ and RO- images. At 7T there is appreciable distortion in multi-echo images and TOPUP correction does provide a subtle increase in sharpness in the RMS images in the worst-affected temporal lobe regions. Note that at 7T there is increased T2* signal dropout in the regions of high B0 inhomogeneity and that vessels are visible in the difference maps that could confound distortion correction.
Discussion
MPRAGE with interleaved reversed RO polarity can provide oppositely distorted images without confounding motion between acquisitions and TOPUP can be used to correct the distortion. The scan times are extended and here we used R=3 GRAPPA to accelerate the scan, except at 3T where the echo 1 of R=3 multi-echo MPRAGE had low SNR. The interleaved RO approach could be useful for highly-accelerated WAVE CAIPI8 that uses ~200 Hz/px readout bandwidth per pixel. Multi-echo MPRAGE was found to be robust to readout distortion even at 7T.Conclusions
Interleaved readout polarity MPRAGE allows for estimation of distortion without motion confound between oppositely distorted images. This approach could be useful for single echo MPRAGE imaging with ~200 Hz/px.Acknowledgements
We are grateful for the following funding sources: R01HD093578, R01HD085813, R42CA183150, S10RR023401, S10RR019307, and S10RR023043.References
1. van der Kouwe, Andre J W and Benner, Thomas and Salat David H and Fischl Bruce. Brain morphometry with multiecho MPRAGE. Neuroimage 2008;40:559–569.
2. Frost R, Varadarajan D, Wang H, Andersson JLR, Boyd E, Tirrell L, Diamond B, Morgan L, Stevens A, Polimeni JR, Fischl B, van der Kouwe AJW. Correction of B0 distortion in high-resolution ex vivo brains with reversed polarity acquisitions. In: Proceedings of the 25th Annual Meeting of OHBM, 2019 (abstract Th566).
3. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003;20:870–888.
4. Zahneisen B, Aksoy M, Maclaren J. Simultaneous interleaved blip up/down readout for dynamic off-resonance correction in functional EPI In: Proceedings of the 25th Annual Meeting of ISMRM, 2017 (abstract 5050).
5. Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med 2012;68:389–399.
6. Shen J, Chen Z, Yang J. New FOCI pulses with reduced radiofrequency power requirements. J Magn Reson Imaging 2004;20:531–537.
7. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage 2012;62:782–790.
8. Polak D, Setsompop K, Cauley SF, Gagoski BA, Bhat H, Maier F, Bachert P, Wald LL, Bilgic B. Wave-CAIPI for highly accelerated MP-RAGE imaging. Magn Reson Med 2018;79:401–406.
Figures