3402
Model-Based Iterative Reconstruction for Echo Planar Imaging: A Preliminary Evaluation for Neuro MRI1Radiology, Mayo Clinic, Rochester, MN, United States, 2Department of Radiologic Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand
Synopsis
A recently proposed model-based iterative
reconstruction (MBIR) method enables to prospectively manage B0-inhomogeneity
and gradient-nonlinearity in EPI, and has been demonstrated to generate images
with high SNR and minimal spatial blurring and geometric distortion. However,
the clinical significance and degree of benefit remains undetermined. In this study,
using commodity EPI acquisition protocols for whole-brain imaging, MBIR was radiologically
compared against standard scanner-generated EPI images and post-processed
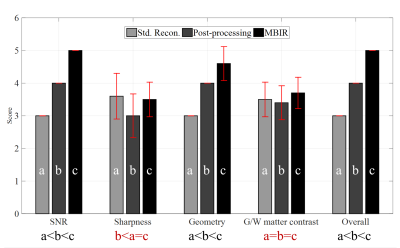
versions. The results show that significant advantage of MBIR (p<0.05) over both the standard scanner-generated
EPI results and post-processed variants was observed in three categories: SNR, geometric
accuracy, and overall image quality.
Purpose
Echo-planar-imaging1,2 (EPI) is widely used clinically for its speed, but is known to be sensitive to non-idealities such as B0 inhomogeneity and gradient nonlinearity. A recently proposed model-based iterative reconstruction3 (MBIR) method prospectively and jointly manages these and other common EPI non-idealities, and has been demonstrated to generate images with high signal-to-noise ratio (SNR) and minimal spatial blurring and geometric distortion. However, the clinical significance and degree of benefit remains undetermined. In this study using commodity EPI acquisition protocols for whole-brain imaging, MBIR was radiologically compared against standard scanner-generated EPI images and post-processed versions of them to characterize its advantage and determine if a broader clinical comparison is warranted.Methods
Ten healthy volunteers were imaged under an IRB-approved protocol and following written informed consent, on a compact 3T MRI scanner4 running GE DV26 (R02) software with a single-shot EPI sequence (voxel size=0.86x0.86x4 mm3, 2x ASSET/SENSE, 5/8 partial Fourier, NEX=2) utilizing an 8-channel brain coil. The images were reconstructed from raw data using the (wavelet) sparsity-regularized MBIR strategy3 which was performed on Matlab2016b, using a 2X-oversampled type-III non-uniform fast Fourier transform (NUFFT) that manages ramp-sampling and gradient nonlinearlity with width=5 Kaiser-Bessel kernel, L=N time segments, and a 7⨯7 shift window. FISTA5 was executed for 30 iterations. The regularization parameter was chosen manually (β=0.002). The default images generated by the scanner using the vendor’s modular reconstruction pipeline6 as well as post-processed (SPM8; unwarp for B0 correction) versions of them, were used for the comparison. Each volumetric study was evaluated under 5 assessment categories (see Fig. 1), each using a 5-point scale (1=worst, 5=best), by 3 fellowship-trained neuroradiologists, in which image scoring was performed in consensus. Wilcoxon signed-rank tests (both one- and two-sided) were used to identify the presence or absence of significant differences in reader scores across three methods.Results
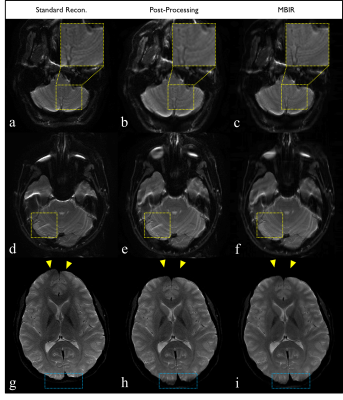
As shown in Figure 1, MBIR received the highest absolute or tied score across all evaluation categories of the evaluated reconstruction methods. Significant advantage of MBIR (p<0.05) over both the standard scanner-generated EPI results and post-processed variants was observed in three categories: SNR, geometric accuracy, and overall image quality. No statistically significant differences were observed for gray/white matter contrast between any methods, which is desirable. MBIR also exhibit significant advantage in terms of sharpness over post-processing results, and no significant difference compared to the standard vendor reconstruction. These trends – and the specific advantages of MBIR over its competitors – are demonstrated in Figure 2. The image-based interpolation (post-processing technique) suffers from fine-structural loss highlighted by the yellow box in 2b, which is visible in the unprocessed vendor (2a) and MBIR (2c) results. Residual aliasing artifacts (i.e. eyeball) from parallel imaging acceleration are also visible on both standard reconstructed image and its post-processed version shown in 2d and 2e, respectively, but are not observed in the MBIR result in 2f. Geometric distortion present in the standard reconstruction (2g) is incompletely corrected by post-processing (2h) but largely mitigate by MBIR (2i). The relatively superior SNR of MBIR is also visually apparent across all slices.Discussions
We described a concerted management of non-uniform k-space sampling, B0 inhomogeneities, gradient nonlinearity, parallel imaging, and image sparsity. The MBIR provides significant improvements in image quality (SNR, geometric accuracy, sharpness) over standard reconstruction/correction methods for conventional EPI acquisition protocols, advantage which may translate into improved diagnostic ability and confidence (e.g., for subtle lesions) without requiring changes to existing clinical protocols or workflow. The underlying strategy of this work is to account for the non-idealities during reconstruction, rather than by a series of corrections in the image domain, which can degrade image fidelity especially when the image is displayed on a relatively coarse pixel grid. A broader investigation into the specific clinical impact of MBIR is thus warranted, and the specific benefits of MBIR for functional MRI (fMRI), diffusion weighted/tensor imaging (DWI/DTI), and MR elastography (MRE) applications will be the further studies.Acknowledgements
This work was supported by NIH U01 EB024450.References
1. Mansfield P. Multi-planar image formation using NMR spin-echoes. J Phys C. 1977; 10:L55–L58.
2. Bernstein MA, King KF, Zhou XJ. Handbook of MRI pulse sequences. Burlington: Elsevier Academic Press; 2004.
3. Yarach , Bernstein MA, Huston J- III, In MH, Kang D, Shu Y, Gray EM, Meyer M, and Trzasko JD. Model-Based Single-Shot EPI Reconstruction with Sparsity Regularization. In Proc. Int. Soc. Magn Reson Med 2019; pp. 0929.
4. Foo TKF, Laskaris E, Vermilyea M, et al. Lightweight, compact, and high-performance 3T MR system for imaging the brain and extremities. Magn Reson Med 2018; 80(5): 2232-2245.
5. Beck A, and Teboulle M. A fast iterative shrinkage-thresholding algorithm for linear inverse problems. SIAM. J. Imaging Science 2009; 2(1): 183-202.
6. Hinks RS, Mock BJ, Collick BD, Frigo FJ, Shubhachint T. Method and system for image artifact reduction using nearest-neighbor phase correction for echo planar imaging. 7,102,352. US Patent. 2006 Sep 5.
Figures