3398
Phantom Development for the Standardization of fMRI Data across Multiple Imaging Sites and Scanners1Graduate School of System Informatics, Kobe University, Kobe, Japan, 2National Institute of Radiological Sciences, National Institutes for Quantum and Radiological Science and Technology, Chiba, Japan, 3Medical Institute of Developmental Disabilities Research, Showa University, Tokyo, Japan, 4Graduate School of Medicine, Osaka University, Suita, Japan, 5Information Science and Technology Center, Kobe University, Kobe, Japan, 6Institute for Quantum Life Science, National Institutes for Quantum and Radiological Science and Technology, Chiba, Japan
Synopsis
Establishing data harmonization in fMRI “big data” is necessary to ensure the reliability and reproducibility of fMRI research. Our group recently developed two distinct phantoms: a brain-shaped phantom to correct field heterogeneity and a functional image-specific phantom to calibrate shape distortion and signal irregularity. A preliminary experiment confirmed a shape distortion and signal irregularity caused by field inhomogeneity when using EPI signals, and these were corrected using an affine transformation. Therefore, our developed phantoms have the potential to contribute to achieving fMRI data standardization.
Introduction
Functional MRI (fMRI) is a major research tool for investigating the neurophysiological status of the brain in both healthy and diseased conditions. In clinical and pre-clinical fMRI research, a great deal of fMRI data are required to achieve reliability and reproducibility, and it is necessary to acquire fMRI data from multiple sites to collect a large amount of data from many volunteers, patients and animal models. However, no method has yet been established to harmonize the fMRI data acquired from multiple imaging sites and scanners, which are characterized by shape distortion and signal irregularity caused by field inhomogeneity and living bodies, such as human and animals, when using an EPI sequence and by differing imaging protocols and parameters. In clinical research, some groups have used travelling human volunteers and patients to standardize and harmonize studies of fMRI data that were acquired from multiple imaging1-4. By contrast, our group focuses on developing special phantoms and compensation algorithms to standardize and harmonize fMRI data acquired from multiple imaging sites. Herein, we describe the development of specific phantoms to correct the shape distortion and signal irregularity caused by field inhomogeneity and by living body when using an EPI sequence.Methods
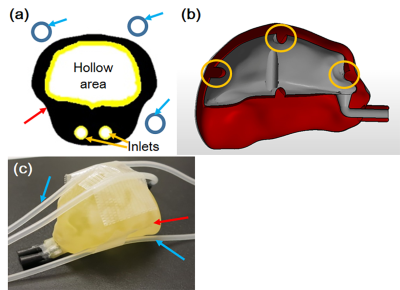
Phantom development: Two types of distinct phantoms were developed for fMRI data harmonization: a brain-shaped phantom to correct field heterogeneity and a functional image-specific phantom to calibrate the shape distortion and signal irregularity caused by living bodies when using an EPI sequence (Fig. 1). The brain-shaped phantom aims to calibrate and correct the shape distortion and signal irregularity caused by field heterogeneity of each scanner, and the functional image-specific phantom aims to compensate for the shape distortion and signal irregularity caused by the living subject (whether animal or human) when using an EPI sequence. In the brain-shaped phantom, the size and shape of the phantom were designed by referring to the size and shape of the target, such as marmoset and mouse, and the phantom was constructed of acrylic resin by a 3D printer. The size and shape of the phantom were adjusted by the size of the coils. A hollow area was created in the brain-shaped phantom, and a copper sulfate aqueous solution was introduced through inlets to evaluate and correct field inhomogeneity (Fig. 1a). To detect the image position, grooves were made on the outer side of the hollow area as position markers (Fig. 1b). In the functional image-specific phantom, the multiple tube-type phantoms with distilled water were used to compensate for the shape distortion and signal irregularity caused by the living subject, such as a marmoset or mouse, because these phantoms can be measured simultaneously with the target living body in fMRI experiments. The phantoms can be installed around the brain-shaped phantom and the target living body5. When functional image-specific phantoms were used with the living body, the positions of the phantoms were used to correct the shape distortion caused by the living body when using an EPI sequence, and the phantoms’ signals were used to compensate for the signal irregularity.Correction of shape distortion: As a preliminary trial for the standardization of fMRI data, an affine transformation compensated for the shape distortion of fMRI data caused by the field inhomogeneity. In this proceeding, the MR images acquired using an EPI sequence were set as fMRI data and spin-echo T2-weighted images were set as standard images. The evaluation criterion for transformation was normalized mutual information.
MR experiments: To evaluate the image quality and preliminary registration using the affine transformation, spin-echo T2-weighted images as a standard image and EPI images as a functional image were acquired using a 7.0 Tesla preclinical MR scanner (Bruker BioSpec 70/20, Bruker BioSpin, Ettlingen, Germany) and an 8-channel phased array coil. The imaging parameters were as follows for the spin-echo T2-weighted images: TR/TE = 2975/33 ms; FOV = 76.8 x 76.8 mm2; matrix size = 128 x 128; slice thickness = 1.0 mm; number of slices = 27; number of acquisitions = 1. The imaging parameters for the EPI images were TR/TE = 2000/18 ms; FOV = 41.4 x 41.4 mm2; matrix size = 69 x 69; slice thickness = 1.0 mm; number of slices = 27; number of acquisitions = 1.
Resutls and discussion
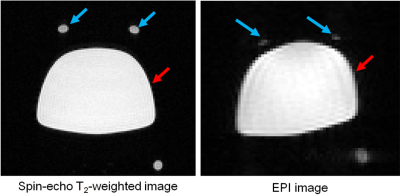
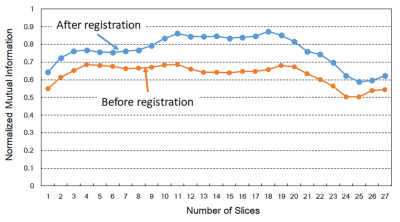
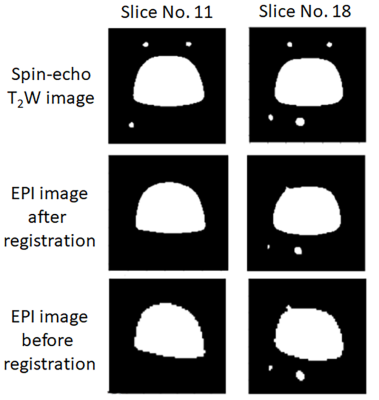
Figure 2 presents the MR images acquired using spin-echo and EPI sequences. The EPI image had a serious shape distortion and signal irregularity in comparison to the spin-echo T2-weighted image. This result clearly indicates that the EPI images are easily affected by field inhomogeneity, demanding a correction procedure. Figure 3 shows the changes to the values of the normalized mutual information before and after registration. The values of the normalized mutual information after registration were always higher than those before registration. The example images after registration, as shown Figure 4, were more similar to the images before registration. Thus, the correction using an affine transformation effectively corrected the influence of field inhomogeneity. In future, we will improve the registration technique for correcting signal disparities caused by field inhomogeneity and living bodies using an EPI sequence.Conclusion
Our developed phantoms may potentially contribute to the standardization of fMRI data.Acknowledgements
This research was supported by AMED under Grant Number JP19dm0307026.References
[1] A. Yamashita, et al: PLoS Biology, 17(4): e3000042, 2019.
[2] C. Hawco, et al: Psychiatry Res Neuroimaging, 282, 134-142, 2018.
[3] O. Mogan, et al: ISMRM 2019, 237, 2019.
[4] W. T. Clarke, et al: ISMRM 2019, 2812, 2019.
[5] D. Kokuryo, et al: Phys. Med. Biol., 55 (14): 4119-4130, 2010.
Figures