3397
ASCII: Acquisition with Self-Correction for Image Inhomogeneity at 7T1Department of Biomedical Engineering, University of Melbourne, Parkville, Australia, 2Melbourne Brain Centre Imaging Unit, University of Melbourne, Parkville, Australia, 3Department of Physical Sciences, Peter MacCallum Cancer Centre, Parkville, Australia, 4Department of Medical Physics and Biomedical Engineering, Shiraz University of Medical Sciences, Shiraz, Iran (Islamic Republic of), 5Center for Neuromodulation and Pain, Shiraz, Iran (Islamic Republic of)
Synopsis
Inhomogeneity of the RF transmit field results in undesirable shading and brightening in high field imaging due to range of flip angles across the imaged object. A new method is proposed whereby two image volumes with coarse resolution in the slice direction are acquired at different flip angles and combined to produce an image volume at higher resolution and free of B1 inhomogeneity. Reconstruction quality is comparable to conventional super-resolution imaging, while image inhomogeneity caused by flip angle error is reduced. This is a flexible method applicable to a range of MRI sequences to correct B1 inhomogeneity.
Introduction
B1 inhomogeneity is a problem for high field imaging which arises due to RF interference and dielectric resonance1. It causes spatial variation in signal intensity which results in brightening and shading in images which can obscure pathology and image features of interest. Attempts to address B1 inhomogeneity have involved application of multiple element transmit arrays2, specialised pulse design3 and post-processing corrections4. Further to this, sequences with some degree of B1 insensitivity implicit in the reconstruction process, such as MP2RAGE5, have gained popularity in high field imaging. Thus we propose an imaging technique where B1 estimation and correction is implicit in the reconstruction process. Acquisition with Self-Correction of Image Inhomogeneity (ASCII) is an auto calibrated imaging technique that combines super-resolution6 and B1 estimation and correction in order to produce image volumes free of B1 transmit bias.Methods
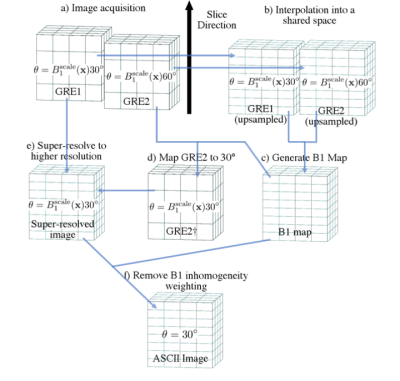
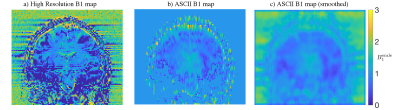
Acquisition:Imaging was performed on a Siemens spherical spectroscopy phantom and in vivo with the same protocol. 2D GRE was acquired with TR=5000ms, TE=10ms, 45 axial slices, in-plane resolution 128x128, FOV 220x220mm (in vivo), FOV 200x200mm (phantom). Two thick (4mm) slice packages were acquired, GRE1 with flip angle=30°, GRE2 with flip angle=60° and 2mm slice offset (Figure 1a). Reference scans were two thin (2mm) slice packages acquired with 90 axial slices, the first with flip angle=30°, the second with flip angle=60°. A reference B1 map was calculated from the thin slice packages using a double angle method7(Figure 2a).
Reconstruction:
The two thick slice packages were interpolated into a shared space (Figure 1b) and following low-pass filtering in the slice direction, a B1 map was extracted using the double angle formula (Figure 2b). The B1 map was used to generate a synthetic image at flip angle = 30° from the flip angle=60° package (Figure 1d).
$$\text{GRE}2\dagger = \text{GRE}1 \frac{\sin \left(\text{B}1^\text{scale}(\mathbf{x})30^\circ\right)} {\sin \left(\text{B}1^\text{scale}(\mathbf{x})60^\circ\right)},$$
with scaling factor $$$\text{B}1^\text{scale}(\mathbf{x}) = \text{B}1(\mathbf{x})/\text{B}1^\text{ref},$$$ where $$$\text{B}1(\mathbf{x})$$$ is the B1 intensity at spatial location $$$\mathbf{x}$$$, compared to a reference B1 intensity $$$\text{B}1^\text{ref}$$$. Super-resolved images with 2mm resolution in the slice direction were computed from GRE1 and GRE2† using Tikhonov-regularised least-squares estimation
$$z = \left(A^TA + \lambda I\right)^{-1}A^Tb,$$
where $$$z$$$ is the high-resolution data, $$$A$$$ is the forward model for acquisition with overlapping thick slices, $$$\lambda$$$ (0.3 for volunteer, 0.05 for phantom) is the regularisation parameter and $$$b$$$ is the interleaved GRE1 and GRE2† data. B1 inhomogeneity weighting was then removed trigonometrically using a 2D low-pass filtered B1 map (Figure 2c) to produce high resolution images free of B1 inhomogeneity (Figure 1e).
Results
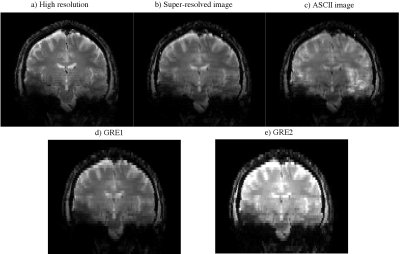
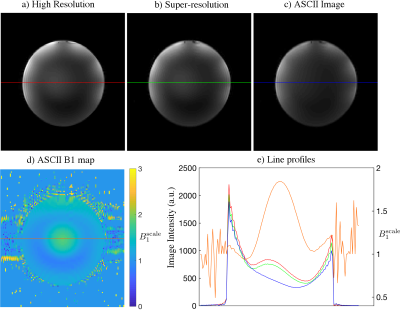
B1 maps produced by ASCII showed the same trend as the conventionally acquired reference B1 map (Figure 2). ASCII produced images with increased resolution in the slice-direction compared to the thick GRE slice packages from which they were reconstructed. ASCII produced an in vivo image volume with similar detail to the reference GRE image with high resolution in the slice-direction while reducing brightening caused by B1 inhomogeneity centrally and around the cortex . Banding artifact which occurred in the centre of the reference GRE image with high resolution in the slice-direction was absent in the ASCII image (Figure 3). In a spherical spectroscopy phantom ASCII reduces the central brightening caused by B1 transmit inhomogeneity (Figure 4).Discussion
ASCII reduces B1 inhomogeneity while reconstructing sub-slice thickness details. This has the potential to increase the utility of super-resolution approaches at high field and allow imaging of fine detailed structure without RF excitation inhomogeneity weighting. Improvement in the B1 estimation step may further reveal obscured details if B1 estimation was extended to be accurate in areas of lower SNR. While the presented data uses 4mm slices for reconstruction, it is expected that use of thinner slices and smaller shifts in the slice direction will allow the slowly varying B1 field to be more accurately mapped. In the presented form ASCII utilises a degree of regularisation which causes some blurring of details, however, increased image sharpness is expected if more sophisticated established super-resolution reconstruction methods are used. While the use of GRE has been sufficient to demonstrate the ASCII concept it has greater potential to improve 2D turbo spin echo8 (TSE) sequences which are highly sensitive to B1 inhomogeneity and have clinical implications, such as T2-FLAIR9.Conclusion
ASCII is a versatile self-calibration technique that has been demonstrated in GRE and has potential to be applied to a range of MRI sequences. It is able to produce B1-insensitive images with scan time and SAR equivalent to sequences that are unable to perform B1 correction without use of an additionally acquired map over the entire volume. Further work will be in optimising ASCII performance, joint estimation of super-resolved images and B1 maps can exploit the slowly spatially varying patterns of B1 transmit fields to more accurately super-resolve overlapping slice packages.Acknowledgements
We acknowledge the facilities, and the scientific and technical assistance of the Australian National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the Melbourne Brain Centre Imaging Unit of the University of Melbourne. The work was also supported by a research collaboration agreement with Siemens Healthineers.References
1. Vaughan, J.T., et al., 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn. Reson. Med., 2001. 46(1): p. 24-30.
2. Lattanzi, R., et al., Electrodynamic Constraints on Homogeneity and Radiofrequency Power Deposition in Multiple Coil Excitations. Magn. Reson. Med., 2009. 61(2): p. 315-334.
3. Garwood, M. and DelaBarre, L., The return of the frequency sweep: Designing adiabatic pulses for contemporary NMR. J. Magn. Reson., 2001. 153(2): p. 155-177.
4. van Schie, J.J.N., et al., Feasibility of a fast method for B-1-inhomogeneity correction for FSPGR sequences. Magn. Reson. Imaging, 2015. 33(3): p. 312-318.
5. Marques, J. P., et al., MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage, 2010. 49(2): p. 1271-1281.
6. Greenspan, H., et al., MRI inter-slice reconstruction using super-resolution. Magn. Reson. Imaging, 2002. 20(5): p. 437-446.
7. Stollberger, R. and Wach, P., Imaging of the active B1 field in vivo. Magn. Reson. Med., 1996. 35(2): p. 246-251.
8. Hennig, J., Nauerth, A. and Friedburg, H., RARE imaging: a fast imaging method for clinical MR. Magn. Reson. Med., 1986. 3(6), p.823-833.
9. Hajnal, Joseph V., et al. "Use of fluid attenuated inversion recovery (FLAIR) pulse sequences in MRI of the brain." Journal of Computer Assisted Tomography, 1992. 16 : p. 841-841.
Figures