3392
Formulating EPI ghost correction as a convolution: Insights into Dual-Polarity GRAPPA by examining kernels of limited extent1Radiology, Brigham and Women's Hospital, Boston, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Department of Radiology, Harvard Medical School, Boston, MA, United States, 5Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
We examine the relationship between traditional 1D linear phase-error correction methods and Dual-Polarity GRAPPA (DPG) for EPI ghost correction. We first show that conventional ghost correction methods based on 1D linear phase errors can be implemented with convolution kernels in k-space, and then generalized in several steps to replicate a typical DPG kernel. We identify that employing multiple phase-encoded lines is important for ghost correction kernel calibration, while incorporating data from multiple channels is critical. Surprisingly, having a kernel extent along the phase-encoded direction is less critical, demonstrating the utility of DPG kernels with limited extent for ghost correction.
Introduction
Ghost correction in echo planar imaging (EPI) is a persistent problem, with multiple proposed solutions[1-5]. The most widely-used solutions employ a linear 1D model to estimate phase errors between data sampled on positive readout gradients, RO+, versus negative readout gradients, RO-, in an EPI echo-train readout. Here, we link conventional linear 1D model ghost correction methods with modern convolution kernel models. To start, we describe how current traditional methods can be implemented using a convolution kernel in k-space. We then expand the kernel extent to include data points along kx and multiple channels to build up a 3D kernel (kx-ky-coil) as proposed in Dual-Polarity GRAPPA (DPG)[3]. The results illustrate observations from the effects of the expanded parameterizations.Theory
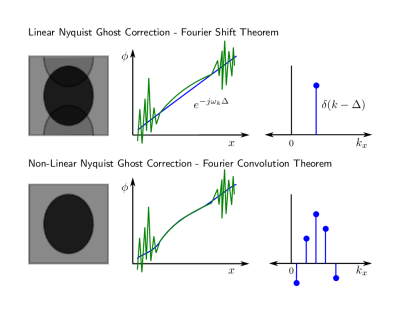
The Fourier Convolution Theorem states that the Fourier transform of a convolution operation and the point-wise multiplication operation of the Fourier transform of each separate element are equivalent $$$\mathscr{F}[f*g]=\mathscr{F}[f]\mathscr{F}[g]$$$. Traditional EPI Nyquist ghost correction (NGC) methods employ a special case, the Fourier Shift Theorem, by modelling shifts along kx in the EPI echo-train readout as a linear phase ramp along x. Traditional methods estimate this shift by comparing spatial domain representations of EPI data acquired with no phase encoding gradients, typically using pre-scan data[1] or using a small number of phase navigator lines prior to each EPI readout[2]. The top row of Fig. 1 illustrates the relationship between a phase ramp in the spatial domain, and a delta function in k-space. EPI, however, is highly susceptible to local field inhomogeneity[3], which can result is a non-linear phase relationship between RO+ and RO-, shown in green. By employing a convolution kernel, one can expand the parameterization as shown in the bottom row of Fig. 1.Methods
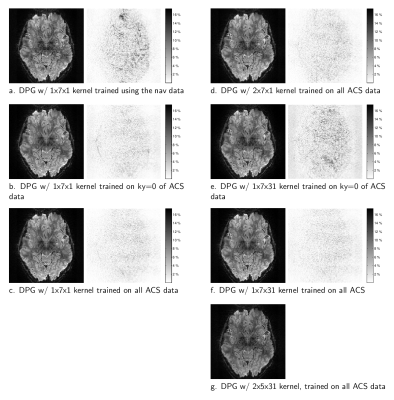
To demonstrate the effect of expanded parameterization, we acquired 1.1mm-isotropic in-vivo 2D EPI data of the human brain with informed consent using a Siemens whole-body 7T MRI scanner equipped with a custom-built 32-channel head-only receive coil array. The protocol parameters were TR=2 s, TE=26 ms, FOV=192 cm, matrix size=174×174, slice thickness=1.1 mm, 34 slices. Calibration data to recover the in-plane sub-sampling rate of R=4 was acquired in a pre-scan using GESTE[6] in order to also generate suitable training data for DPG reconstruction. In addition, 3 phase-navigator lines were acquired prior to each EPI echo train, for alternate reconstruction using LPC[2]. Kernel calibration was evaluated using three data sets: 3 phase navigator lines; the ky=0 line from the pre-scan data; and the complete pre-scan data. DPG ghost correction was performed using a variety of different sized kernels. Missing in-plane data were recovered using a 2ky-by-5kx GRAPPA kernel trained on the ghost-free GESTE image.Results
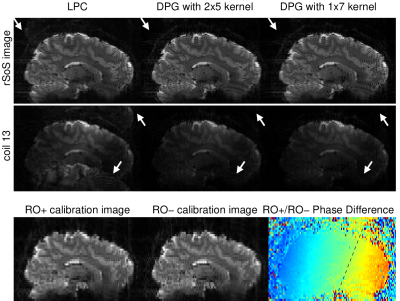
Figure 2 illustrates that using a convolution kernel for LPC-based ghost correction can result in images that are nearly identical to the traditional approach. Here, unique kernels for each coil were generated by a Fourier transform of the LPC 1D linear phase-correction operator, and convolved with the measured data. Limiting the extent of the kernel along kx to 7-points, as in Fig. 2(c), introduces kernel truncation artifacts however. The images presented in Figure 3 illustrate the reconstruction quality from a variety of DPG kernels derived from the 3 different sources of training data. Fig. 3(a) illustrates a kernel derived from the 3 phase-navigator lines, using a kernel size/extent that is identical the LPC kernel above. Note the quality is significantly degraded in comparison to the other reconstructions. However, using a single line of calibration data, at ky=0, from the pre-scan data greatly reduces ghosting artifacts Fig. 3(b). Ghosting artifacts are further reduced if one uses all of the calibration data available, Fig. 3(c). Expanding the kernel parameterization, we see that the extent along ky provides little improvement, Fig. 3(d), whereas employing multiple coils greatly improves the image quality, Fig. 3(f), if there is sufficient calibration data to train on (comparing Fig. 3(e) to Fig. 3(f)).Although the 1x7x31 DPG kernel in Fig. 3(f) provides surprisingly good image quality, we expect DPG kernels need extent along ky correct 2D phase-errors. To test this, we reconstructed data from a known scenario[7] where 2D phase errors exist. Surprisingly, Fig. 4 illustrates that even in the presence of 2D phase errors, a DPG kernel of limited extent still outperforms LPC, suggesting that local field inhomogeniety degrades the LPC estimate itself.
Conclusion
This work examined the relationship between traditional NGC methods and modern convolution kernel methods. We first cast the traditional LPC method as a convolution kernel, and then expanded the parameterization in discrete steps to build up to DPG. We demonstrated that deriving DPG kernels from 3-line navigator data is not viable, and that the inclusion of phase encoded calibration data is important for DPG. The most important parameterization, however, is to employ a kernel that extends across multiple coils. We conjecture that this provides a broader signal subspace from which to support the estimation of ghost-free data, rather than the alternate possibility that the improvement stems from SNR gains due to more training data. The results show both that kernel extent along kx or ky is less critical, even in cases where 2D phase errors between RO+ and RO- are present.Acknowledgements
This work was supported in part by the NIH NIBIB (grants P41-EB015896, R01-EB019437 and R03-EB023489,), by the BRAIN Initiative (NIH NIMH grant R01-MH111419), and by the MGH/HST Athinoula A. Martinos Center for Biomedical Imaging; and was made possible by the resources provided by NIH Shared Instrumentation Grant S10-RR019371.
References
- Ahn CB, Cho ZH. A new phase correction method in NMR imaging based on autocorrelation and histogram analysis. IEEE Trans on Medical Imaging 1987;6(1):32–36.
- Feiweier T. Magnetic resonance method and apparatus to determine phase correction parameters, 2013. US Patent 8,497,681.
- Hoge WS, Polimeni JR. Dual-polarity GRAPPA for simultaneous reconstruction and ghost correction of EPI data. Magn Reson Med 2016;76(1):32–44.
- Lobos RA, Kim TH, Hoge WS, Haldar JP. Navigator-free EPI ghost correction with structured low-rank matrix models: New theory and methods. IEEE Transactions on Medical Imaging 2018;37(11):2390–2402.
- Lee J, Han Y, Ryu JK, Park JY, Ye JC. k-Space deep learning for reference-free EPI ghost correction. Magnetic Resonance in Medicine 2019;82(6):2299–2313.
- Hoge WS, Tan H, Kraft RA. Robust EPI Nyquist ghost elimination via spatial and temporal encoding. Magn Reson Med 2010;64(6):1781–1791.
- Hoge WS, Setsompop K, Polimeni JR. Dual-polarity slice-GRAPPA for concurrent ghost correction and slice separation in simultaneous multi-slice EPI. Magn Reson Med 2018;80(4):1364–1375.
Figures