3386
Optimized gradient spoiling for B1 mapping with UTE AFI that is less sensitive to the RF-spoiling phase increment
Marta Brigid Maggioni1, Martin Krämer1, and Jürgen R. Reichenbach1,2,3,4
1Medical Physics Group, Institute of Diagnostic and Interventional Radiology, Jena University Hospital - Friedrich Schiller University, Jena, Germany, 2Michael Stifel Center for Data-driven and Simulation Science Jena, Friedrich Schiller University, Jena, Germany, 3Abbe School of Photonics, Friedrich Schiller University, Jena, Germany, 4Center of Medical Optics and Photonics, Friedrich Schiller University, Jena, Germany
1Medical Physics Group, Institute of Diagnostic and Interventional Radiology, Jena University Hospital - Friedrich Schiller University, Jena, Germany, 2Michael Stifel Center for Data-driven and Simulation Science Jena, Friedrich Schiller University, Jena, Germany, 3Abbe School of Photonics, Friedrich Schiller University, Jena, Germany, 4Center of Medical Optics and Photonics, Friedrich Schiller University, Jena, Germany
Synopsis
B1 mapping is a major challenge for reliable T1 quantification, and it is especially difficult for UTE sequences, because most conventional B1 mapping methods are unable to perform on short T2* tissues. Recently, an implementation of the actual flip angle imaging (AFI) method was proposed for UTE sequences. The AFI method requires ideal spoiling of residual transverse magnetization, which is typically achieved by fine-tuning RF-spoiling phase increments as well as the strength of spoiler gradients. In this work, we propose an improved gradient spoiling scheme, which reduces the effect of the RF-spoiling phase increment on the AFI results.
Introduction
The uncertainty in the estimation of the flip angle caused by inhomogeneities of the B1 field is a major source of unreliable T1 quantification when using methods based on flip angle variation (VFA). Although several methods have been proposed for directly mapping the B1 distribution1,2, most of them cannot be applied to tissues with short T2* relaxation because such tissues require ultrashort echo-time (UTE) imaging sequences in order to retrieve signal. Recently, an actual flip angle (AFI) B1 correction method was proposed for UTE sequences3. The AFI technique uses a sequence with two interleaved repetition-times (TR). The signal from both TRs is then combined, to generate a B1 map. The AFI method relies on a pulsed steady-state regime, which, however, has been shown to be highly sensitive to variations of the RF-spoiling phase increment (φ) as well as the amplitude and duration of the spoiler gradients4,5. Various optimal values for φ have been proposed in literature3,4,5 but they depend on the choice of TRs, and a small variation in φ can also produce significant errors in B1 estimation4,5,6. Therefore, very long spoiler gradients with high amplitudes have been proposed as methods for eliminating the dependence on φ5. In combination with 3D radial hard-pulse excitation-based UTE sequences this comes at the cost of significant increases of the acquisition time. In this work, we propose an optimized gradient spoiling scheme, which reduces the dependency of UTE AFI based B1 mapping on φ and allows a reduction of the spoiling gradient duration and thus the total acquisition time.Methods
A hard-pulse 3D radial UTE sequence7 was extended by adding two adjustable interleaved TRs for AFI (Fig. 1) and further modified to allow for a wide range of φ values in combination with adjustable durations and amplitudes of the spoiler gradients. For the gradient spoiling scheme, constant spoiling in z-direction was always used (after rephasing the magnetization back to the center of k-space). In addition, the sequence allowed for random spoiler gradients in the x- and y-directions. The purpose of the latter was to further “scramble” the transverse magnetization to achieve, on average, a more pure steady-state signal. The sequence was applied to a 12cm diameter spherical phantom, filled with water and superabsorber polymer for stabilization. Different TR combinations as well as amplitudes and durations of the gradients spoilers were chosen (Table 1) and tested on 180 phase increment values ranging from 1° to 180°, with all other acquisition parameters kept identical: flip angle 45°, isotropic resolution (3.5×3.5×3.5)mm3. All measurements were performed with a clinical 3T MRI scanner (PRISMA, Siemens) using the vendor supplied single-channel transmit/receive knee coil. The method was further validated to correct the T1 quantification results on a knee of a healthy 35-year-old male volunteer. The T1 map was calculated by using a variable flip angle (VFA) approach (FAs: 5°,8°,10°,12°,15°,20°,25°,30°,35°,40°, TR: 5 ms, (1.4×1.4×3.0)mm³ anisotropic resolution), and corrected by using an AFI sequence with the same resolution, FA:45°,TR:15 ms and AG1/AG2 of 65/325, where AG is the spoiler gradient area expressed in [mT·ms/m].Results
Figure 2 shows the calculated flip angle correction factor for a spherical region of interest in the centre of the phantom for the 180 RF-increments φ with different spoilers and TR combinations. Even the measurement with the largest spoiler gradient amplitude and duration, but spoiling only in the z direction (red curve) still showed a dependency on φ, that is only increased with the application of smaller gradient spoilers (blue curve). The introduction of randomized spoiling in the x and y direction, however, not only is able to completely eliminate the dependency on φ, but is also able to achieve comparable correction factors, even with a reduction of the amplitude of the gradients and the TRs (yellow, violet and green curves). Figure 3 shows the T1 VFA map before and after correcting for B1 inhomogeneities with the AFI correction, the results of T1 quantification in regions of interest (ROI) in the tendon, bone marrow and muscle are shown in table 2.Discussion and Conclusion
By adding randomized x and y spoiling to a 3D UTE AFI sequence we demonstrated that the dependency on the RF-spoiling phase increment can be highly reduced, potentially making UTE AFI B1 mapping more robust. Investigating why the added randomized x and y gradient spoiling stabilizes the AFI results would require full Bloch simulations of the AFI sequence, including the initial phase where the steady-state is reached. We suspect that the added randomized spoiling effectively adds a scrambling of the residual transverse magnetization to the sequence which then reduces potential for refocusing of old magnetization (that would disturb the steady-state signal required for AFI). This, in combination with the strong k-space center averaging in a 3D center-out radial acquisition of the UTE sequence, ultimately stabilizes the steady-state signal. The proposed improved gradient spoiling scheme, furthermore, has the advantage of allowing a reduction in the duration and amplitude of the spoiler gradients and thus also TRs, ultimately leading to a reduction of the total acquisition time. Furthermore, the in-vivo results of the proposed AFI correction lead to T1 results that are in line with literature8,9.Acknowledgements
No acknowledgement found.References
- Cunningham CH, Pauly JM, Nayak KS. Saturated double-angle method for rapid B1+ mapping. Magn Reson Med 2006;55:1326–1333.
- Chung S, Kim D, Breton E, et al,Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout, Magn Reson Med. 2010;64(2):439–446.
- Nehrke K, On the steady‐state properties of actual flip angle imaging (AFI). Magn. Reson. Med., 2009; 61: 84-92.
- Yarnykh V L, Actual flip‐angle imaging in the pulsed steady state: A method for rapid three‐dimensional mapping of the transmitted radiofrequency field. Magn. Reson. Med., 2007; 57: 192-200
- Yarnykh V L, Optimal radiofrequency and gradient spoiling for improved accuracy ofT1and B1measurements using fast steady‐state techniques. Magn. Reson. Med., 2010; 63: 1610-1626
- Ma Y, Lu X, Michael C, et al. Accurate T1 mapping of short T2 tissues using a three‐dimensional ultrashort echo time cones actual flip angle imaging‐variable repetition time (3D UTE‐Cones AFI‐VTR) method. Magn. Reson. Med, 2018; 80-89.
- Herrmann KH, Krämer M, Reichenbach JR Time Efficient 3D Radial UTE Sampling with Fully Automatic Delay Compensation on a Clinical 3T MR Scanner, PLOS ONE, 2016, 11(3)
- avala Bojorquez J, Bricq S, Acquitter C, et al, What are normal relaxation times of tissues at 3 T?, Magnetic Resonance Imaging, 2017; 35, 69-80.
- Ma Y, Zhao W, Wan L, et al.Whole knee joint T1 values measured in vivo at 3T by combined 3D ultrashort echo time cones actual flip angle and variable flip angle methods. Magn Reson Med., 2019; 81:1634–1644
Figures
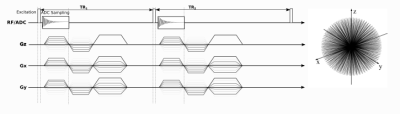
Fig 1: Sequence diagram for AFI B1 mapping.
The sequence is characterized by two interleaved TRs (TR1
and TR2)
with constant spoiler gradients along the z direction and randomized
gradients along the x- and y-direction. Rephasing and spoiling steps
are drawn separately in the diagram for better clarity, whereas in
the actual imaging sequence they were merged. On the right, a
representation of the 3D center-out trajectories in k-space is shown.
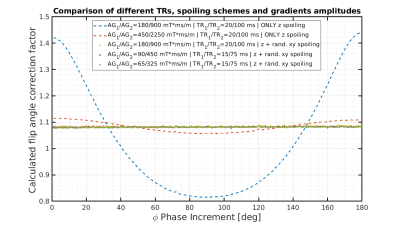
Fig 2: Calculated flip angle correction factor as
a function of the phase shift increment of the RF- spoiling. The
dashed curves were acquired with only constant spoiler gradients
along the z direction while the dotted ones were acquired with
additional randomized spoiling along the x and y directions. The
different TRs and amplitude of the gradients used are specified in
the legend
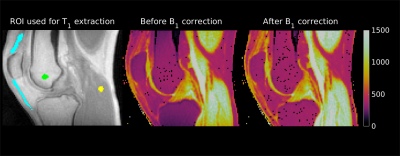
Fig 3: T1 maps before and after the AFI correction of
the volunteer’s knee (middle and right). Both maps
are scaled between 0 ms and 1500 ms. The image on the left shows the
ROIs used to extract the values of the T1 relaxation
times, overlaid on the magnitude data.
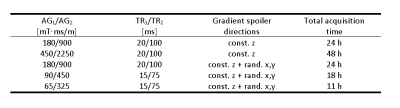
Table
1: Summary table of the most relevant acquisition parameters for the
measurements performed in the phantom.

Table
2: Summary table of the T1 relaxation
parameter values for some selected ROIs in the knee before and after AFI B1 correction.