3365
Optical prospective motion correction enables improved quantitative susceptibility mapping (QSM) of the brain at 7T1Stanford University, Stanford, CA, United States, 2Cornell University, Ithaca, NY, United States, 3GE Healthcare, San Francisco, CA, United States
Synopsis
Recent literature has shown the potential of high‐resolution quantitative susceptibility mapping (QSM) with ultra‐high field (7T) MRI for investigating the magnetostatic properties of brain structures and disease pathology. Higher spatial resolutions, however, require longer scans resulting in a higher likelihood of subject movement. Here, we apply a novel prospective real-time optical motion tracking and correction system using a camera integrated between the Tx and Rx coils of a commercial 7T head coil to demonstrate the feasibility of acquiring high-resolution R2* and QSM images robust to subject motion.
Introduction
Quantitative susceptibility mapping (QSM) has shown potential in elucidating novel insights into the pathology associated with traumatic brain injury, multiple sclerosis, Alzheimer’s disease, and other diseases of the brain. Because susceptibility‐related frequency differences scale linearly with field strength, and noise in the GRE phase scales inversely proportional to the signal magnitude, performing QSM on ultra-high-field (7T) MR systems may facilitate further discoveries. However, addressing motion artifact is a pivotal step in translating QSM to 7T systems. Subject motion can cause spurious phase fluctuations as well as image shifts that generate inconsistencies between the measured field and the bulk magnetic susceptibility of the underlying tissue. Prior work has shown that prospective motion correction (PMC) can provide improved quantitative susceptibility maps,1 but this work used large bore-mounted cameras, limiting line-of-sight on systems that use local closed-shell transmit head coils such as the Nova 7T head coil. Our prior work2 implemented an optical prospective motion correction system at 7T with a coil-integrated camera with line-of-sight to a marker placed on the subject’s forehead which addresses this issue. The goal of the present study is to test this fully integrated optical PMC system on high-resolution QSM at 7T. This is expected to enable more widespread use of QSM at 7T, facilitating discovery of novel neuroimaging biomarkers.Methods
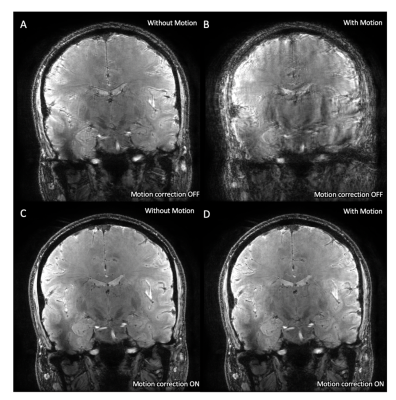
The prospective tracking system utilized here consisted of an MR‐compatible camera, designed and built to be mounted between the transmit (Tx) and receive (Rx) coils of a Nova Medical 2-channel Tx/32-channel Rx head coil, a marker, and a tracking computer which supplies motion updates at each TR.2 This set-up enables direct line-of-sight to an MR-safe, checkerboard-marker placed with adhesive on the subject’s forehead allowing the real-time tracking of subject motion during scanning. All experiments were conducted on a 7T GE MR950 scanner.To demonstrate proof-of-concept of this PMC system for in vivo 7T QSM applications, we acquired a 3D coronal multi-echo gradient echo (mGRE) (0.35x0.45x1.5mm resolution, matrix size = 512x384x146, 4 TEs (6.54-27.31 with an echo spacing of 6.92ms), TR = 34ms, scan time 7min27s) four times on a single healthy, compliant subject: no motion with and without correction, and with deliberate motion (discrete rotations ~10-15 degrees to the left or right every 45 seconds) with and without correction.
For each experimental condition, we performed a voxel-by-voxel fitting of the signal vs. echo time to compute R2* maps, and also used the morphology enabled dipole inversion (MEDI) method3 to compute quantitative susceptibility maps. All images were reconstructed at 0.35x0.35x1.5mm resolution.
Results
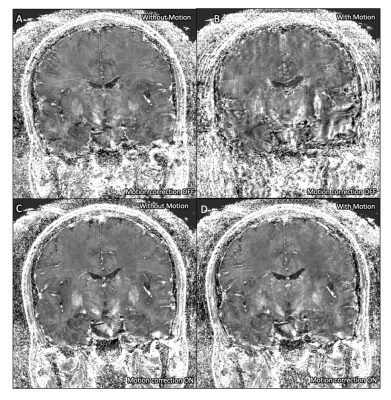
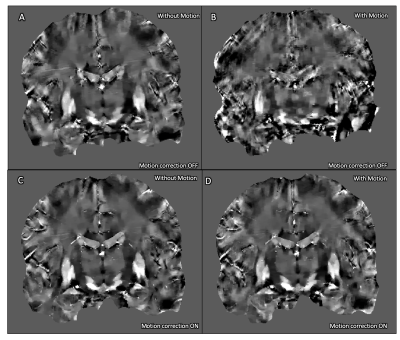
The magnitude images acquired without deliberate rotational motion show minor motion artifact without correction (Fig. 1A), visibly improved in the corrected acquisition (Fig. 1C). Deliberate motion without correction resulted in poor and almost unusable image quality (Fig. 1B). This was significantly improved with motion-correction, enabling visualization of deep-gray and brainstem nuclei (Fig. 1D).A similar trend was seen in the R2* maps (Fig. 2) and QSM maps (Fig. 3). The uncorrected acquisitions resulted in motion artifact, ranging from minor in the acquisitions without deliberate rotational motion (Fig. 2A, 3A) to severe in the acquisitions with deliberate motion (Fig. 2B, 3B), with the corrected acquisitions showing demonstrable visual improvement (Fig. 2C-D, 3C-D).
Discussion
Motion-correction for both deliberate and involuntary motion during an mGRE was successfully demonstrated on human subjects, producing high-quality R2* and QSM images at 7T. Qualitative visual improvement in the corrected acquisitions was demonstrated. Future work will add quantitative assessment of image quality and will extend the validation of this PMC system for QSM to additional subjects across a range of ages and disease conditions. Additionally, because PMC is correcting for bulk changes in head position and orientation, but not for changes of the head relative to the magnetic field, the effect of orientation-dependent changes in field perturbation on the present QSM results needs to be further explored.Conclusion
Our motion-correction system demonstrates the potential to overcome motion artifact at 7T, one of the biggest challenges precluding the utilization of ultra-high-field MR for QSM. Motion artifact was present even in a healthy, compliant subject, demonstrating the vulnerability of these sequences to subject motion and underscoring the importance of this approach. PMC allows for ultra-high-resolution QSM and R2* mapping that is robust to subject motion, which may facilitate discovery of novel biomarkers of aging and disease in the brain. We hope to utilize this technique to analyze QSM changes across hippocampal subfields during the progression of Alzheimer’s disease and other neurodegenerative diseases.Acknowledgements
The authors would like to acknowledge research support by GE Healthcare, NIH P41 EB015891, NIH S10 RR026351-01A1, and ASNR Boerger Alzheimer’s Fund, NIH R01 1R01AG061120-01.References
1. Mattern H, Sciarra A, Lüsebrink F, Acosta‐Cabronero J, Speck O. Prospective motion correction improves high‐resolution quantitative susceptibility mapping at 7T. Magnetic Resonance in Medicine. 2018.
2. DiGiacomo P, Tong E, Maclaren J, Aksoy M, Bammer R, Rutt B, Zeineh M. A novel, coil-integrated camera for prospective optical motion correction of brain imaging at 7T. International Society of Magnetic Resonance in Medicine. 2019, Montreal, Canada.
3. Liu J, Liu T, de Rochefort L, Ledoux J, Khalidov I, Chen W, Tsiouris AJ, Wisnieff C, Spincemaille P, Prince MR, Wang. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage. 2012.
Figures