3362
The impact of through-plane motion on 2D FISP-MR Fingerprinting: a simulation study based on realistic patient and healthy volunteer movements1High Field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Christian Doppler Laboratory for Clinical Molecular MR Imaging, MOLIMA, Vienna, Austria
Synopsis
Motion-induced artifacts in quantitative MRI may not be visually detectable. MRF has shown to be robust to in-plane movement, and methods to mitigate it were made available. However, the impact of clinically prevalent motion patterns has not yet been investigated. The effect of realistic motion data from patients with neurodegenerative disorders and young/elderly controls on 2D FISP-MRF is therefore investigated in this simulation study. Results are validated with in-vivo motion-tracked measurements. Although through-plane motions were shown to significantly impact MRF parametric maps (particularly T2), MRF may preserve its sensitivity in clinical cases in which expected lesion-related differences are large enough.
Introduction
Motion artifacts in MRI are often easily discernable as blurring or ghosting. In parametric maps derived with quantitative MRI, however, these may manifest as an unrecognizable bias1.Even though resilience to in-plane motion has been shown in Magnetic Resonance Fingerprinting (MRF)2, studies have suggested that 2D-MRF is rather sensitive to significant through-plane motion3.4.
This study investigates the impact of clinically representative motions patterns in 2D FISP-MRF5 in a simulation setting. Results are validated with volunteer measurements accompanied by real-time motion tracking.
Methods
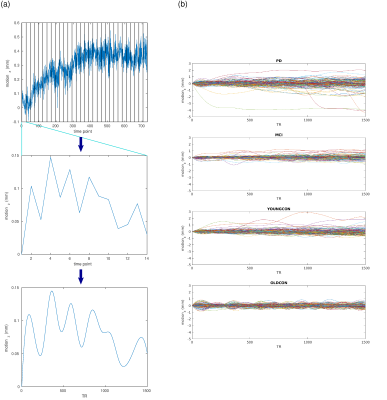
1D-through-plane motion was implemented in MATLAB code simulating the behavior of a prototype 2D FISP-MRF5 sequence with a prescan-based6 B1+ correction and spiral sampling with a spiral angle increment of 82.5°.7 This was attained by assuming a linearly distributed ensemble of spins aligned along the z-axis, which was displaced in slice direction, proportional to the magnitude of the input motion. A constant B1+ field equal to 1 was assumed for simplicity. Input T1 and T2 values for gray (GM) and white matter (WM) were extracted from ROIs (in the frontal lobe) of previous healthy volunteers MRF measurements (same sequence). MRF signals were calculated using a Bloch simulation. These (motion-corrupted) complex signals were matched to a dictionary derived using the same simulation assuming no motion, and differences between resulting relaxation parameters and those not affected by motion were calculated.The set of motion data included in the 1D simulation was derived as follows: Motions from four groups of participants (n=147) were collected in previous studies using a sequence containing 3D EPI-based navigators for prospective motion correction8. Participants comprised patients with Parkinson’s Disease (PD; n=44) and Mild Cognitive Impairment (MCI; n=38) as well as young (YOUNGCON; n=31) and old (OLDCON; n=34) controls scanned using head restraints9. Motion logs spanned a period of ~20 minutes (TR=1.6s) and were split into segments of 20s (i.e., the acquisition time of one MRF slice) resulting in 22,638 motions of 20s duration. Each motion segment was interpolated to the MRF temporal resolution using cubic splines to resemble a smooth (realistic) transitional movement. These motions were input into the 1D simulations (Figure 1).
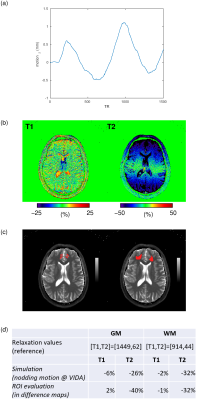
For validation of the simulation results, a healthy volunteer was scanned on a 3T whole-body scanner (MAGNETOM Vida, Siemens Healthcare, Erlangen, Germany) using a prototype implementation of the 2D FISP-MRF5 sequence described above, with undersampling factor 48, 1500 TRs per slice, FOV 256mm, resolution 1.0x1.0x5.0mm3. The volunteer was instructed to execute a continuous slow nodding motion around the left-right axis throughout the acquisition of a single brain slice (~20s). Motion was recorded with an in-bore camera (KinetiCor Inc., Honolulu, HI, USA). For comparison purposes, a scan with no intentional motion was acquired in addition. Pure through-plane motion was derived at the position of the ROIs (Figure 3(c)) from the motion log by combining translation and rotation uniquely along z and input into the simulation pipeline to determine theoretical changes in relaxation parameters. Difference maps were calculated with reference to no motion. ROIs in GM and WM were manually drawn to assess motion-induced differences in in-vivo parametric measures. Changes in relaxation parameters were compared between simulation and in-vivo measures.
Results
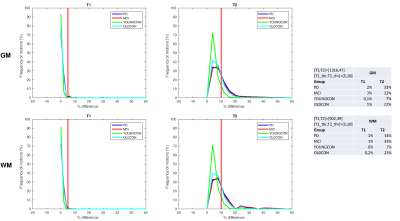
Figure 2 displays frequency line histograms of simulated motion-induced T1 and T2 relaxation times changes calculated for GM and WM. A low percentage of patient motions (up to 3%) resulted in T1 changes in both GM and WM by more than 5%. T2 relaxation times, however, showed higher susceptibility to through-plane motion. 7% of the motions in YOUNGCON and up to 34% in the PD and MCI groups, as well as 22-23% of the motions in OLDCON, resulted in a >10% T2 change.As illustrated in Figure 3(b), T1 values were largely insensitive to a through-plane nodding motion. The opposite is observed for T2 values, where differences of >40% may be apparent with a <2mm motion amplitude. Even though substantial large differences were observed in GM, changes in WM relaxation times derived by simulation were in excellent agreement with ROI changes (Figure 3(d)).
Discussion/Outlook
Substantial motion-induced differences in MRF parametric maps could potentially impair detection of lesion-related alterations in clinical populations. However, studies employing MRF e.g. in brain tumor patients have shown that lesion-related alterations in relaxation values are greater than the changes that could be induced by the investigated motions here10,11.A slice thickness of 5mm was used throughout this study. However, investigation of smaller lesions (e.g. MS) may require thinner slices (~3mm), where an even larger susceptibility to motion is expected. In such studies, head restraints are mandatory, but cannot prohibit through-plane motion completely.
Simulations containing synthetically derived typical motion patterns occurring at different time points of the MRF acquisition are planned for investigating the sequence’s temporal sensitivity to motion. Validation of these will be attempted on a phantom with realistic brain relaxation times and in-vivo with motion tracking.
Conclusion
Clinically prevalent through-plane motions were shown to have a significant impact in 2D FISP-MRF parametric maps (particularly T2). However, depending on the extent of expected lesion-related changes, MRF may preserve its sensitivity in most of the cases. Nevertheless, particularly for studies investigating subtle quantitative changes, the development of motion detection and correction strategies will be essential.Acknowledgements
This study was funded by the Austrian Science Fund (FWF) project KLI679.We are grateful to Drs. Dan Ma, Yun Jiang, and Mark Griswold for sharing the Bloch simulation code.
References
[1] Callaghan M. F. et al., An evaluation of prospective motion correction (PMC) for high resolution quantitative MRI, Front Neurosci. 2015; 9: 97
[2] Ma D. et al., Magnetic resonance fingerprinting, Nature. 2013; 495: 187–192
[3] Mehta G. et al., Image reconstruction algorithm for motion
insensitive MR Fingerprinting (MRF): MORF, MRM. 2018; 80(6): 2485-2500
[4] Yu Z. et al., Exploring the sensitivity of magnetic resonance
fingerprinting to motion, MRI. 2018; 54: 241-248
[5] Jiang Y. et al., MR fingerprinting using fast imaging with
steady state precession (FISP) with spiral readout, MRM. 2015; 74(6): 1621-1631
[6] Chung S. et al., Rapid B1+ mapping using a preconditioning RF
pulse with TurboFLASH readout, MRM. 2010; 64(2): 439-446
[7] Körzdörfer G. et al., Effect of spiral undersampling patterns
on FISP MRF parameter maps, MRI. 2019; 62: 174-180
[8] Bogner W. et al., 3D GABA imaging with real-time motion
correction, shim update and reacquisition of adiabatic spiral MRSI, NeuroImage. 2014; 103: 290-302
[9] Heckova E. et al., Real-time Correction of Motion and Imager
Instability Artifacts during 3D γ-Aminobutyric Acid–edited MR Spectroscopic
Imaging, Radiology. 2018; 286(2): 666-675
[10] Badve et al., MR Fingerprinting of Adult Brain Tumors:
Initial Experience, AJNR. 2017; 38(3): 492-499
[11] De Blank et al., Magnetic Resonance Fingerprinting to
Characterize Childhood and Young Adult Brain Tumors, Pediatr. Neurosurg. 2019; 54: 310-318
Figures