3356
Prospectively corrected dual-echo EPI using optical tracking1Department of Radiology, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States, 2Brigham and Women's Hospital and Harvard Medical School, Boston, MA, United States, 3Advanced Clinical Imaging Technology, Siemens Healthcare, Lausanne, Switzerland, 4Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 5École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 6Siemens Healthcare, Erlangen, Germany
Synopsis
Large head motion induces different local magnetic-field inhomogeneities, even if the field of view is corrected prospectively. In this work, we implemented and evaluated a diffusion-weighted dual-echo EPI sequence that prospectively corrects for motion using real-time measurements from an optical tracker and uses echoes acquired with reversed phase-encoding to correct for the distortions resulting from the induced local magnetic field inhomogeneities. We evaluated our motion and distortion correction framework in volunteer experiments undergoing controlled motion, and in pediatric patients undergoing routine MRI. Prospective motion correction using our proposed method produced high-quality diffusion parameter maps in all volunteer and patient scans.
Introduction
Head motion during MRI acquisition reduces image quality and increases the overall cost of MRI1,2. This is a particularly challenging problem in diffusion weighted MRI (DW-MRI) as DW-MRI relies on encoding the amount and direction of the movement of water molecules, which is highly sensitive to patient motion. Many retrospective3,4,5 and prospective6,7,8,9 methods have been proposed to tackle this problem but the adaptation of these techniques to clinical practice has been limited, especially in the case of uncooperative populations such as young children. Most of these methods suffer from either slow pose update rates, and/or uncorrected local magnetic field changes that are exacerbated by large motion. In this work, we propose and evaluate a solution that uses highly accurate and fast motion measurements from an optical tracker to prospectively update the FOV, and acquires a second echo10 with opposing phase-encoding direction to enable the estimation and correction of motion-induced field changes.Methods
All the sequence development and MR image acquisition was performed at 3T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with an in-bore optical tracking system (Kineticor Inc). A product spin-echo EPI sequence was modified to add a second readout matching the first readout in terms of resolution, FOV and bandwidth, but with reversed k-space traversal in the phase-encoding direction. Optical tracking motion measurements were used to update the FOV using a motion-correction (MoCo) module in the sequence. The FOV was updated continuously during the scan except during the EPI readouts. The sequence was tested on controlled-motion volunteer studies and pediatric patients undergoing clinical MRI. All subjects provided written informed consent.Volunteer studies: Four volunteers (age 28-38; 2 females, 2 males) were scanned. For all the volunteer studies, a standard 30-direction single shell diffusion weighting with a b-value of 1000s/mm2 (b1000) and five images without any diffusion encoding gradients (b0) were acquired. Sequence parameters were TE1/TE2=72/108 ms, GRAPPA=2, TR=12000 ms, FOV=256 mm, 2-mm isotropic resolution with 70 slices, resulting in a scan time of 6 minutes. For comparison, a single echo acquisition would have TR=9300ms with these settings. A T2-weighted FSE sequence was also acquired to provide a structural reference. Four sets of data were acquired on each volunteer: 1) No motion with noMoCo, 2) No motion with MoCo, 3) Motion with noMoCo and 4) Motion with MoCo. For the motion cases, volunteers were asked to move their head to a different position using audio cues every minute.
Patient studies: Five pediatric patients (age 7-17, 1 female 4 males) were scanned. In addition to the clinical fast diffusion protocol, a dual-echo prospectively corrected scan with matching sequence parameters was acquired. Six-direction diffusion tensor imaging with a b-value of 1000s/mm2 and one image without any diffusion encoding gradients were acquired. Sequence parameters were TE1=72ms, TE2=108ms, GRAPPA=2, TR=8000 ms, 2 mm in plane resolution with forty 4-mm slices, resulting in a scan time of 1:40 minutes. Images were reconstructed offline and fed into a distortion correction algorithm using FSL’s topup11. Fractional anisotropy (FA) maps were calculated for each diffusion scan using in-house developed software.
Results
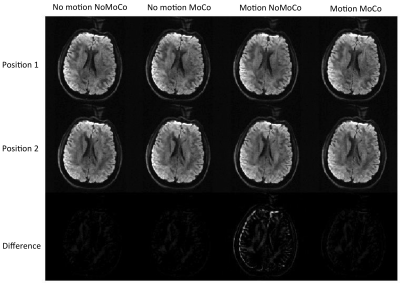
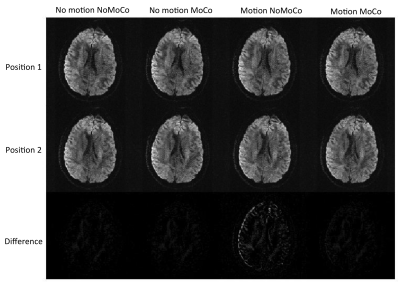
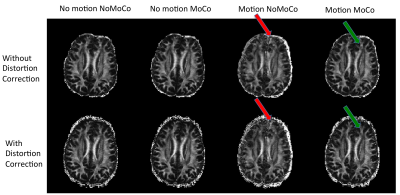
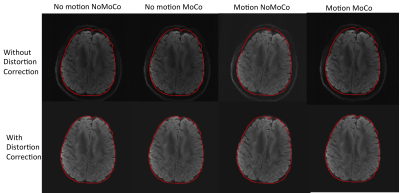
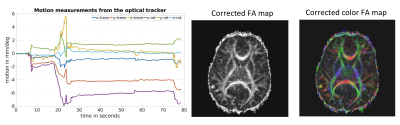
Figure 1 shows an example from a volunteer study, where axial slices for the first echo are shown from the first b1000 volume and the last b1000 volume. Figure 2 shows the same data for the second echo. Motion correction did not produce additional artifacts when the subject was not moving and improved the results significantly when the subject was moving. Figure 3 shows the resulting FA maps from each set, with and without the proposed distortion correction strategy. The RMSE between the ground truth (no motion; noMoCo) and the other three sets were 2.56±1.21 (no motion; MoCo), 8.44±3.21 (motion; noMoCo) and 3.21±1.55 (motion; MoCo) percent over the 4 volunteers in the distorted corrected set. The maximum motion was 6mm/deg and mean motion was 1.8mm/deg over all 4 volunteer motion scans. Figure 4 shows the brain boundary, calculated from a structural scan overlaid on DW-MRI images. Motion and distortion corrected images show a much better alignment when the subject is moving. Figure 5 shows example diffusion parameter maps from an 11-year-old male patient, with the motion trace. Our correction resulted in reliable parameter estimates even when the subject moved up to 8mm/deg.Discussion and Conclusion
Our results demonstrate that prospective motion correction worked reliably on all the volunteer cases. When there was no motion, the results were equivalent to the no correction scans. When there was motion the RMSE was substantially improved compared to no motion-correction. As can be seen from Figure 3, the distortion correction further improves the results of the prospective motion-correction algorithm. Although the prospective motion-correction algorithm reliably moves the FOV to the correct position, due to the different local magnetic field changes, different head positions inside the bore result in different distortion, creating artifactual changes in FA. This effect is most clearly visible in the areas close to large susceptibility changes, such as in the frontal lobes near the sinuses. Our initial results in patients also show that this technique is promising and can be used to improve the clinical diffusion MRI acquisitions in pediatric populations. More clinical validation is needed to establish the efficacy of the method.Acknowledgements
This research was supported in part by the following grants: NIH-R01EB019483, NIH-R01NS079788, NIH-R01EB018988, and a pilot grant (PP-1905-34002) from National Multiple Sclerosis Society.References
1) Andre, J.B., Bresnahan, B.W., Mossa-Basha, M., Hoff, M.N., Smith, C.P., Anzai, Y. and Cohen, W.A., 2015. Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations. Journal of the American College of Radiology, 12(7), pp.689-695
2) Afacan, O., Erem, B., Roby, D.P., Roth, N., Roth, A., Prabhu, S.P. and Warfield, S.K., 2016. Evaluation of motion and its effect on brain magnetic resonance image quality in children. Pediatric radiology, 46(12), pp.1728-1735
3) Kober, T., Gruetter, R. and Krueger, G., 2012. Prospective and retrospective motion correction in diffusion magnetic resonance imaging of the human brain. Neuroimage, 59(1), pp.389-398
4) Holdsworth, S.J., Aksoy, M., Newbould, R.D., Yeom, K., Van, A.T., Ooi, M.B., Barnes, P.D., Bammer, R. and Skare, S., 2012. Diffusion tensor imaging (DTI) with retrospective motion correction for large‐scale pediatric imaging. Journal of Magnetic Resonance Imaging, 36(4), pp.961-971
5) Rohde, G.K., Barnett, A.S., Basser, P.J., Marenco, S. and Pierpaoli, C., 2004. Comprehensive approach for correction of motion and distortion in diffusion‐weighted MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 51(1), pp.103-114
6) Benner, T., van der Kouwe, A.J. and Sorensen, A.G., 2011. Diffusion imaging with prospective motion correction and reacquisition. Magnetic resonance in medicine, 66(1), pp.154-167
7) Herbst, M., Maclaren, J., Weigel, M., Korvink, J., Hennig, J. and Zaitsev, M., 2012. Prospective motion correction with continuous gradient updates in diffusion weighted imaging. Magnetic resonance in medicine, 67(2), pp.326-338
8) Aksoy, M., Forman, C., Straka, M., Skare, S., Holdsworth, S., Hornegger, J. and Bammer, R., 2011. Real‐time optical motion correction for diffusion tensor imaging. Magnetic resonance in medicine, 66(2), pp.366-378
9) Alhamud, A., Taylor, P.A., van der Kouwe, A.J. and Meintjes, E.M., 2016. Real-time measurement and correction of both B0 changes and subject motion in diffusion tensor imaging using a double volumetric navigated (DvNav) sequence. NeuroImage, 126, pp.60-71
10) Gallichan, D., Andersson, J.L., Jenkinson, M., Robson, M.D. and Miller, K.L., 2010. Reducing distortions in diffusion‐weighted echo planar imaging with a dual‐echo blip‐reversed sequence. Magnetic resonance in medicine, 64(2), pp.382-390
11) Andersson, J.L., Skare, S. and Ashburner, J., 2003. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage, 20(2), pp.870-888
Figures