3348
MR compatible 4D Ultrasound based validation of respiratory motion compensation strategies1Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2GE Healthcare, University of Wisconsin-Madison, Madison, WI, United States, 3Department of Electrical Engineering, Stanford University, Stanford, CA, United States, 4Department of Electrical Engineering, University of California-Berkely, Berkeley, CA, United States, 5Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 6Department of Engineering Physics, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Imaging during respiratory and cardiac motion remains a major challenge for body and cardiac MR. A number of motion correction approaches have been proposed to address this challenge including use of external gating signals, navigator acquisitions, and motion estimation from the image data itself. However, the accuracy of these motion estimation techniques has not been validated in-vivo. In this work, we use a recently developed MR compatible 4D ultrasound probe combined with feature tracking to evaluate the performance of MR based navigation algorithms in the setting of free-breathing 3D pulmonary imaging.
Introduction
Imaging in the presence of physiological motion including respiratory and cardiac motion has remained a major challenge for body and cardiac MR. High resolution free-breathing 3D imaging has shown potential for new paradigms in assessment of structure and function. These 3D techniques, however, typically rely on respiratory gating or correction strategies to achieve high fidelity reconstructions (4). A number of motion correction approaches have been proposed including use of external gating signals (8), acquisition of pencil beam navigator data (9), and motion estimation from the imaging data itself (self navigators). Use of self navigation for motion estimation is a particularly attractive approach because unlike pencil beam navigators, there is no need to interrupt the imaging sequence. However, questions remain regarding the accuracy of self navigators and other motion estimation techniques in-vivo (4). This is particularly true for developing self-navigation techniques which use constrained reconstructions to provide dynamic 3D volumes, rather than 1D respiratory correlated signals. For free breathing 3D imaging to be adopted clinically, methods are needed for validating, optimizing, and comparing motion estimation techniques in-vivo against a gold standard. In the clinical setting, 4D ultrasound (US) provides high frame-rate volumetric imaging with real-time, in-vivo positional tracking independent of the MR acquisition. We propose leveraging a recently developed MR compatible 4D ultrasound probe (1,2) to evaluate the performance of MR based navigation algorithms in the setting of free-breathing 3D pulmonary imaging (5).Methods
Four healthy volunteers were imaged using simultaneous 3T MRI (Signa Premier, GE Healthcare, Waukesha, WI) and 4D US (GE Vivid E95, GE Healthcare, Milwaukee, WI) with an MR compatible probe. US Images were collected during free-breathing simultaneously with collection of a 3D radial UTE MR acquisition and sampling of a respiratory belt signal. The US probe was placed with an intercostal window over the dome of the liver. MR Imaging parameters include: TE/TR=0.08/3.1ms, 1.25mm isotropic resolution, full lung coverage, ~60k projections, scan time=3:06, high channel density RF coil (GE Air Coil). Respiratory information was extracted from the 3D radial acquisition using DC signal navigation and a recently proposed dynamic 3D image navigator (4,6). For the DC signal extraction, a low pass filter (0.5 hz cut-off frequency) was applied to the center of k-space through time, followed by median filtering of the raw data, and an asymmetric least squares method to remove baseline drift (4). 3D UTE data was reconstructed in a time resolved fashion using a memory efficient multi-scale low rank reconstruction (6) producing a dynamic 3D image with 310ms temporal resolution. Reconstructions were GPU accelerated (SigPy) and performed at 2.5mm isotropic spatial resolution. To allow comparison to other techniques, a respiratory surrogate was extracted using 3D block matching of a location centered on the dome of the liver. A similar approach was used for the ultrasound data where each 3D block was centered around a vascular feature of interest in the liver (3). See figure 1 for a visual description of this workflow. The respiratory bellows signal, DC-based navigators, and dynamic 3D self navigators signals were all compared against ultrasound based volumetric feature tracking. These motion estimation signals were compared visually and quantitatively using pair-wise correlation coefficients. Finally, these estimates were used in a full resolution, respiratory phase resolved reconstruction using L1 wavelet + differences time iterative SENSE.Results and Discussion
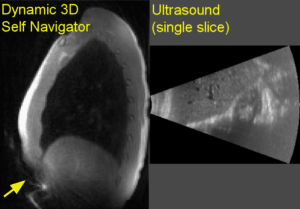
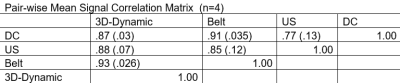
Figure 2 shows example slices from simultaneous collected 4D ultrasound and dynamic 3D self navigators. Respiratory signals derived from the various motion estimates appear by visual inspection to remain in phase with the ultrasound derived motion estimate throughout the MR acquisition (figure 3). Pair-wise correlations averaged across n=4 volunteers agree with this finding (figure 4). Although statistically significant differences between signals were not assessed given the study’s small sample size, it is interesting to note that greater variance in signal correlation was observed between ultrasound measurements and indirect motion estimate techniques (self gating and respiratory bellows) than the 3D dynamic navigator which estimates motion directly from a block around the liver edge. Figure 5 shows images from a typical volunteer generated with the respiratory resolved reconstruction using gating based on the different motion estimates. No visually apparent differences were seen between reconstructions using different gating signals.Conclusions
In this feasibility study in lean healthy volunteers, motion estimation techniques were generally found to agree with ultrasound. This demonstrates the ability to achieve comparable gating results across all methods in a relatively ideal setting. However challenges with DC and respiratory gating methods are well known, including baseline shifts, insensitivity to bulk motion, and lack of direct correlation to organ displacement. Previous studies have shown improved reconstruction performance using self navigators versus other motion estimation techniques in obese patients and patients with structural lung disease. Work is ongoing to utilize the approach we have outlined here to better test and validate self navigator performance in these populations (4).Acknowledgements
The authors wish to acknowledge support from NIH R01CA190298, NIH/NHLBI HL 136965, GE Healthcare, and a Research and Development Grant from the Departments of Radiology and Medical Physics, University of Wisconsin.
References
1.A. Foo TKF, et al. Proc. 26th ISMRM, p. 4416.
2.B. Lee W, Chan H, Chan P, Fiorillo T, Fiveland E, Foo T, Mills D, Patel A, Sabatini J, Shoudy D, Smith S, Bednarz B, A magnetic resonance compatible E4D ultrasound probe for motion management of radiation therapy. IEEE Intl Ultrasonics Symp 2017; doi: 10.1109/ultsym.2017.8092223.
3.C. Shepard AJ, Wang B, Foo TKF, Bednarz B, A block matching based approach with multiple simultaneous templates for the real‐time 2D ultrasound tracking of liver vessels. Med Phys 2017; 44: 5889-900.
4.Jiang, Wenwen, et al. "Motion robust high resolution 3D free‐breathing pulmonary MRI using dynamic 3D image self‐navigator." Magnetic resonance in medicine 79.6 (2018): 2954-2967.
5.Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med 2013; 70: 1241– 1250.
6.Ong, Frank, and Michael Lustig. "Beyond low rank+ sparse: Multiscale low rank matrix decomposition." IEEE journal of selected topics in signal processing 10.4 (2016): 672-687.
7.Luo J, Addy NO, Ingle RR, Baron CA, Cheng JY, Hu BS, Nishimura DG. Nonrigid motion correction with 3D image‐based navigators for coronary MR angiography. Magn Reson Med 2016; 77: 1884– 1893.
8. Runge VM, Clanton JA, Partain CL, James Jr AK. Respiratory gating in magnetic resonance imaging at 0.5 T. Radiology 1984;151:521–523.
9. Ehman RL, Felmlee JP. Adaptive technique for high-definition MRimaging of moving structures. Radiology 1989;173:255–263.
Figures