3347
MR-Based Cardio-Respiratory Motion Correction of Simultaneously Acquired PET Images Through Coil Fingerprints1New York University, New York, NY, United States, 2Siemens Healthineers, Erlangen, Germany, 3Siemens Medical Solutions, New York, NY, United States, 4European Institute for Molecular Imaging, Munster, Germany, 5Radiology, New York University, New York, NY, United States
Synopsis
We present of a real-time, motion correction methodology for MR/PET acquisitions. Our approach utilizes self-refocused navigators that could be integrated into any pulse sequence for providing cardio-respiratory motion information after each excitation. We demonstrate that this approach improves small lesion detection sensitivity and quantitative accuracy for simultaneously acquired MR/PET scans.
Introduction
The simultaneous nature of MR/PET data acquisition presents unique opportunities for the correction of cardiac and respiratory motion blur. Previous MR/PET cardio-respiratory motion correction (MoCo) schemes use a navigated motion-sensitive sequence to assign motion states (gates) to the amplitude of a one-dimensional (and sometimes two-dimensional through the use of EKG leads) motion-tracking signal and retrospectively reorder the PET mode data (1,2). Such approaches are only effective when the motion is periodic (negligible changes in minimum/maximum amplitude) and the PET signal is stationary (which excludes dynamic studies). As these conditions are not routinely met (40% of subjects are irregular breathers(3)and respiratory motion is not symmetric) these approaches have been proven of limited effectiveness and cannot achieve optimal signal-to-noise ratio (SNR) as unassigned counts are discarded. We present a workflow-efficient approach for the reduction of cardio-respiratory blur and partial-volume bias from PET body scans based on the use of self-refocused navigators (SRN's) that could be incorporated into any pulse sequence. We demonstrate that this approach is capable of improving small lesion detection sensitivity and the quantitative accuracy of PET images concurrently acquired with sequences equipped with such navigator modules.Methods
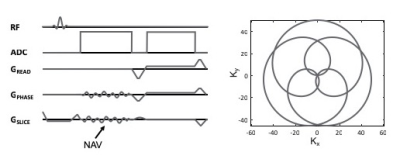
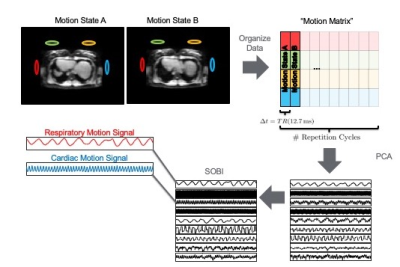
Data sets were acquired on an anthropomorphic phantom as well as human subjects using an integrated MR/PET scanner (Biograph mMR, Siemens Healthineers, Erlangen, Germany). All imaging protocols included the use of a calibration sequence (figure 1) where self-refocused rosette navigators (4) are used generate a tracking signal that can be used to identify cardiac and respiratory motion sates from a Principal Component Analysis (PCA) and Second Order Blind Identification (SOBI) analysis of the navigator signal (figure 2). Because the navigators are self-refocused, their use does not change the steady state of the magnetization and they can, therefore, be used with any imaging sequence at the expense of a minimal increase in the excitation TR (1-2ms). PET images were reconstructed using dynamic deformations of the lines of response (LOR’s) based on motion fields calculated between each cardiac/respiratory motion state and a single reference state. The attenuation maps were, likewise, transformed according to the deformation fields determined from the motion states. Both phantom scans and in vivo scans used the same data acquisition, analysis and reconstruction approaches.Results
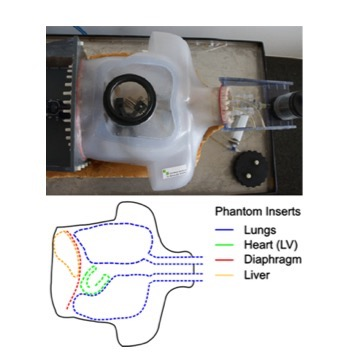
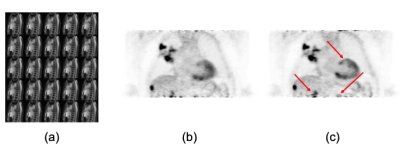
Figure 3 presents a photograph and schematic depiction of the phantom use for validating the accuracy of our approach. The phantom contains several compartments (Lungs, Heart, Liver, Diaphragm) that can be filled with MR and/or PET “contrast” and “moved” according to a prescribed time series. The motion of the phantom is pneumatically actuated and sensors record the actual displacement of the diaphragm as a function of time. Lesions (see below) of known size can be affixed to any of these compartments and thereby estimate the presence/removal of motion-related signal bias before/after motion correction. Figure 4 demonstrates the accuracy of the proposed motion correction approach. In this figure, a small vial (1cc volume) with FDG was affixed to the diaphragm while MR and PET data were simultaneously acquired during “Static” (ground truth) and “Dynamic” acquisitions. The coil fingerprint data from a dynamic stack of stars acquisition (TR=12.7ms, 800 spokes, 37 partitions, 256x256x48 matrix size) were used to obtain respiratory tracking signals from which a motion-distorted system matrix was generated. Clearly, when compared to the static image (figure 4a), the conventional PET reconstruction (figure 4b) suffers greatly from motion blur. This motion blur is completed eliminated from the lesion when the modified (motion-corrected) system matrix is used (figure 4c). Figure 5 presents in vivo results that document the quality of the detected motion states (5a) as well as the PET image quality before (5b) and after (5c) motion correction. These results illustrate significant improvements in small lesion detection sensitivity as well as standard uptake value (SUV) quantification as a result of the removal of motion blur.Discussion
MR/PET has distinct advantages for the assessment of metabolism as a result of PET’s high sensitivity and MR’s high spatial and temporal resolution. The combination of these features to achieve high sensitivity with improved spatial and temporal resolution necessitates the use of information from MR in order to compensate the effects of motion blur and partial voluming effects from the PET images. MoCo is a necessary step when considering these advantages in the abdominal cavity and the approach presented here has been demonstrated to overcome most of the limitations that previous implementations of MR-based MoCo suffered from. The small increase in sequence TR associated with the use of the SRN’s does not appear to have major limitations for the use of the proposed technique during routine clinical practice and its compatibility with all imaging sequences using for abdominal scans would enable the use of the PET data acquired during an entire MR/PET examination.Conclusions
Coil fingerprints via SRN's provide an effective means to track cardiac and respiratory abdominal motion during a concurrent MR/PET examination. The robustness of the SRN signal, its analytical form (which allows optimizing its motion sensitivity) and its compatibility with all imaging sequences used for abdominal MR/PET examinations make this approach very attractive for routine clinical use.Acknowledgements
This work was supported in part by PHS grant P41 EB017183.References
1. Catana C. Motion Correction Options in PET/MRI. Semin Nucl Med. 2015;45(3):212-23.
2. Wurslin C, Schmidt H, Martirosian P, Brendle C, Boss A, Schwenzer NF, Stegger L. Respiratory motion correction in oncologic PET using T1-weighted MR imaging on a simultaneous whole-body PET/MR system. J Nucl Med. 2013;54(3):464-71.
3. Liu C, Pierce LA, 2nd, Alessio AM, Kinahan PE. The impact of respiratory motion on tumor quantification and delineation in static PET/CT imaging. Phys Med Biol. 2009;54(24):7345-62.
4. Rigie D, Vahle T, Zhao T, Czekella B, Frohwein LJ, Schäfers K, Boada FE. Cardiorespiratory motion-tracking via self-refocused rosette navigators. Magn Reson Med. 2019; 81(5):2947-2958.
Figures