3346
A Synthetic Combination of Accurate De-enhanced Registration and Dynamic Artificial Sparsity for Robust High-Resolution Liver DCE-MRI1School of Biomedical Engineering, Guangdong Provincial Key Laboratory of Medical Image Processing, Southern Medical University, Guangzhou, China, 2Department of Radiotherapy, Cancer Center, Guangdong Provincial People's Hospital & Guangdong Academy of Medical Science, Guangzhou, China, 3Shandong Medical Imaging Research Institute, Shandong University, Jinan, China, 4School of Computer and Information Science, Hubei Engineering University, Wuhan, China, 5Division of Superconducting Magnet Science and Technology, Institute of Electrical Engineering, Chinese Academy of Sciences, Beijing, China, 6Department of Biomedical Engineering, Zhejiang University, Hangzhou, China, 7School of Information Technology and Electrical Engineering, The University of Queensland, Brisbane, Australia
Synopsis
High spatiotemporal DCE-MRI is a valuable tool in liver disease diagnoses and treatments. Recently, there is a growing research trend which focuses on the motion-robustness of liver DCE-MRI. However, current techniques cannot simultaneously solve the motion problem when pursuing high spatiotemporal resolution. In this work, we propose to combine an accurate registration technique with dynamic artificial sparsity for high spatiotemporal resolution DCE-MRI of liver. The experiments indicated that the proposed framework results in better image quality than iGRASP due to de-enhanced image registration. Compared to motion-sorting techniques, the proposed framework generates better temporal resolution.
Target Audience
Radiologist and scientists who take an interest in real-time abdominal MRI and high spatiotemporal motion-free DCE-MRIPurpose
DCE-MRI is an emerging tool for diagnoses and treatments of liver diseases 1. During liver DCE-MRI scanning, subject involuntary movements including respiratory and cardiac motion, stomach and bowel peristalsis are inevitable 2, 3. Recently, there has been a substantial increase of research activity in the field of motion-robustness liver DCE-MRI 4–9. Previous studies used golden-angle radial acquisition to reduce motion artifacts, which attracts a lot of attentions from academia and clinic 4–6. However, it’s not enough for motion-robustness liver DCE-MRI. For example, iGRASP and L+S average the motion in all directions, resulting in image blurring of anatomical details 4, 5. Motion-sorting techniques 7–9 resolve the motion problem to some extent by motion state separation at the sacrifice of temporal resolution, which impairs its ability of accurate DCE-MRI parameter study. The aim of this work is to propose a robust abdominal DCE-MRI technique which not only alleviates the motion problem, but also provides high spatiotemporal resolution. Specifically, a robust registration scheme is incorporated into the dynamic artificial sparsity technique to achieve this goal. Compared to iGRASP-style methods, the proposed scheme provides motion-free results. Meanwhile, the imaging results indicate that the proposed technique is more robust, and preserves temporal resolution better than motion-sorting type techniques.Methods
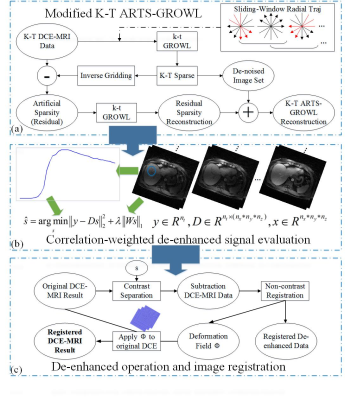
The proposed framework consists of three parts: dynamic artificial sparsity reconstruction scheme 6, correlation-weighted de-enhanced signal evaluation 10 and a registration operator based on intensity-invariant residual complexity 11. The complete framework is displayed as Fig.1.Initially, a dynamic artificial sparsity scheme (i.e., k-t ARTS-GROWL) is used to obtain the image reconstruction result 6. In this study, a sliding-window radial GROWL is employed to further improve the SNR of the reconstructed image comparing to original k-t ARTS-GROWL, as shown in Fig.1(a).
Subsequently, the correlation-weighted sparse signal s recovery procedure is performed as the following:
$$\tilde{s}=argmin\left \| y-Ds \right \|_2^2+\lambda \left \| Ws \right \|_1$$
Where y is the ideal signal-intensity time courses averaged in certain liver ROI, D is an over-complete dictionary which contains all the signal-intensity curves in the image series, W is the correlation factor which is related to the dictionary.
In the registration step, an intensity-invariant residual complexity with B-splined-based free-form deformation is employed in this work 11. To be more specific, a deformation field Φ is estimated primarily using de-enhanced DCE-MRI result in the previous step by MIRT toolbox 12. Then, the accurate deformation field Φ is applied to the original artificial sparsity result to obtain the motion-free DCE-MRI.
Experiments
An in vivo liver DCE-MRI experiment was performed on a 3.0 T Prisma MR scanner (Siemens AG Medical Solutions, Erlangen, Germany) using a 20-channel body/spine coil array. A 3D stack-of-stars FLASH pulse sequence with free-breathing golden-angle radial sampling scheme was employed for this acquisition. The relevant parameters are listed as follows: FOV=350 × 350 × 240 mm3, TR/TE = 3.6/1.6 ms, number of slices 12, number of readout points in each spoke 512, oversampling ratio 2, number of spokes 1144, and slice thickness 5 mm.In this work, each time frame was constructed by 34 consecutive spokes in all cases, all computations (dynamic artificial sparsity, 2d or 3d registrations) were implemented in Matlab (R2014a; the Mathworks, Natick, MA, USA), for off-line reconstruction on an HP workstation (12 Core/2.10 GHz, 128GB, Intel Xeon E5-2620 v2 CPU).
Data Analysis
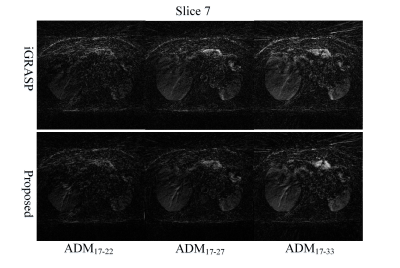
In this study, we came up with the concepts of adjacent difference map (ADM) and its corresponding relative root-mean-square error (rRMSE) in post-contrast phase of DCE-MRI, which were calculated in the following to exam the effect of motion correction.$$ADM_{i-j}=\left | I_i^{post-contrast}-I_j^{post-contrast} \right |(i\not\equiv j)$$
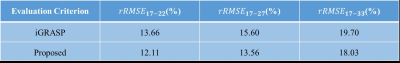
$$rRMSE_{i-j}=\left \|I_i^{post-contrast}-I_j^{post-contrast} \right \|_F/\left \| I_i^{post-contrast} \right \|_F(i\not\equiv j)$$
here $$$I_i$$$ and $$$I_j$$$ represent the ith and jth time frame, respectively.
Results & Discussion
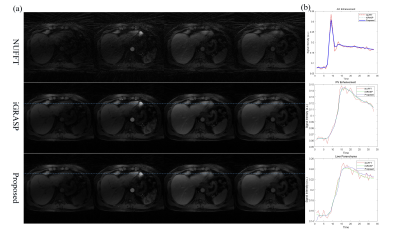
The results of liver DCE-MRI from different approaches were shown in Fig.2. The corresponding ADM results were displayed in Fig.3. It can be clearly seen that the proposed framework has smaller difference between adjacent frames than that of iGRASP, especially in ADM17-33. The relative RMSE values in Table 1 also support this observation. From difference maps, we can see motion correction performs well in the proposed scheme. The movements between different frames in iGRASP were dramatically removed using the proposed scheme without temporal resolution loss (Fig. 2(b), Fig. 3). This is important for DCE-MRI parameter estimation.Because XD-GRASP type techniques impair the temporal continuity of DCE-MRI data, we do not compare our results with those approaches. In addition, the proposed framework is very flexible, and can be easily extended to all DCE-MRI acquisitions, while the motion-sorting techniques can only be used for some certain type trajectories. Moreover, the proposed framework will have better performance than XD-GRASP in dealing with aperiodic motions.
Conclusion
The proposed scheme is a registration-based dynamic artificial sparsity framework for high spatiotemporal resolution DCE-MRI of liver. Compared to iGRASP-type techniques, it offers advantages such as high image quality, effective elimination of motion effect and improvement of the accuracy of perfusion parameters. Compared with motion-sorting techniques, it offers better temporal resolution, which is useful in clinical DCE-MRI application.Acknowledgements
This work was supported by NSFC grant (61801205) and CPSF grant (2018M633073). We thank Dr. Li Feng from Icahn School of Medicine at Mount Sinai for his constructive suggestions.References
[1] Aronhime S, Calcagno C, Jajamovich GH, et al. DCE-MRI of the liver: effect of linear and nonlinear conversions on hepatic perfusion quantification and reproducibility. J Magn Reson Imaging. 2014; 40(1): 90–98.
[2] Wood ML, Henkelman RM. MR image artifacts from periodic motion. Med Phys. 1985; 12(2): 143–151.
[3] Lin W, Guo J, Rosen MA, et al. Respiratory Motion-Compensated Radial Dynamic Contrast-Enhanced (DCE)-MRI of Chest and Abdominal Lesions. Magn Reson Med. 2008; 60(5): 1135–1146.
[4] Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014; 72(3): 707–717.
[5] Otazo R, Candès E, Sodickson DK. Low-rank plus sparse matrix decomposition for accelerated dynamic MRI with separation of background and dynamic components. Magn Reson Med. 2015; 73(3): 1125–1136.
[6] Chen Z, Kang L, Xia L, et al. Sequential combination of parallel imaging and dynamic artificial sparsity framework for rapid free-breathing golden-angle radial dynamic MRI: K-T ARTS-GROWL. Med Phys. 2018; 45(1): 202–213.
[7] Feng L, Axel L, Chandarana H, et al. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med. 2016; 75(2): 775–788.
[8] Chen Z, Kang L, Xu Z, et al. MR ARTS-GROWL: A Non-Iterative Motion-Resistant Technique for High Spatiotemporal Liver DCE Imaging. ISMRM, Honolulu, USA, 2017. p. 3208.
[9] Feng L, Huang C, Shanbhogue K, et al. RACER-GRASP: Respiratory-weighted, aortic contrast enhancement-guided and coil-unstreaking golden-angle radial sparse MRI. Magn Reson Med. 2018; 80(1): 77–89.
[10] Zhou Y, Sun Y, Yang W, et al. Correlation-weighted sparse representation for robust liver DCE-MRI decomposition registration. IEEE T Med Imaging. 2019; doi: 10.1109/TMI.2019.2906493.
[11] Myronenko A, Song X. Intensity-based image registration by minimizing residual complexity. IEEE T Med Imaging. 2010; 29(11): 1882–1891.
[12] https://sites.google.com/site/myronenko/research/mirt.
Figures