3344
High efficiency Free-Breathing 3D Thoracic Aorta Imaging with Self-Navigated Image Reconstruction1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2Shenzhen College of Advanced Technology, University of Chinese Academy of Sciences, Shenzhen, China
Synopsis
Self-gating techniques can be used to solve and compensate for cardiac or respiratory motion during MRI with free-breathing. In this work, a self-gating based motion correction scheme is proposed, and combined with a 3D variable-flip-angle TSE sequence for high-efficiency thoracic aorta imaging. Specifically, the slab-selective SPACE sequence is modified to acquire self-gating signals, which are used for detecting the respiratory motion. The image data is subsequently corrected based on the binning motion correction and image registration approach. The comparison was conducted on healthy volunteers and compared against a conventional diaphragmatic navigator-gated acquisition to assess the feasibility of the proposed scheme.
Introduction
Three-dimensional (3D) black-blood MRI is a promising noninvasive imaging technique for assessing aortic atherosclerotic plaque 1. However, respiratory motion remains a major problem in 3D aortic imaging. Respiratory gating technique is employed to acquire data only when the respiratory signal coincides with a predefined acceptance window. This technique suffers from low acquisition efficiency and has a long scan time. Recent studies have shown that self-gating techniques can be used to solve and compensate for cardiac and respiratory motion during MR imaging with free-breathing 2. In this work, a novel self-gating based motion correction scheme is proposed, and combined with a 3D variable-flip-angle turbo spin-echo sequence (SPACE) for high-efficiency free breathing thoracic aorta imaging. Specifically, the slab-selective SPACE sequence is modified to acquire self-gating signals, which are used for detecting the respiratory motion. The image data is subsequently corrected based on the binning motion correction and image registration approach 3. This work was evaluated through in-vivo experiment and compared against a conventional diaphragmatic navigator-gated acquisition to assess the feasibility of the proposed framework.Methods
Sequence and Image Reconstruction: The 3D T1-weighted black-blood SPACE sequence is modified to include two additional readouts in the superior-inferior (SI) direction, immediately before the imaging readouts (Figure 1). The Fourier transform of these lines is the 1D projection of the entire image volume, which is called the self-gating (SG) lines. The respiratory motion is estimated using the correlation calculation with the average SG lines, which is also used for correcting the respiratory motion artifacts in the SI direction. Then the acquired data is retrospectively assigned to Nbins respiratory bins according to their positions in the breathing cycle, and outlier data attributed to deep breaths are removed. Each bin data is reconstructed using the L1-regularized iterative SENSE soft-binning approach 4 by solving Equation (1):$$\widehat{I_{n}}=argmin_{I_{n}}||W_{n}(AI_{n}-K_{n})||_2^2+\lambda||\psi I_{n}||_{1} (1)$$
$$ n=1⋯N_{bins} $$
$$ W=\begin{cases}e^{-\alpha(d-thresh)} & d > thresh\\1 & otherwise \end{cases}(2)$$
Where A represents the MR system matrix including the receiver coil sensitivities, the Fourier transform, and the sampling pattern.$$$\widehat{I_{n}}$$$ are the reconstructed images, $$${W_{n}}$$$ is the data weights for the n-th bin , d represents the estimated SI respiratory motion with respect to the end of expiration. $$${K_{n}}$$$ is the data acquired at each respiratory bin after 1D translational motion correction 3,5. The second term enforces sparsity by using the L1-norm of the wavelet coefficients of $$${I_{n}}$$$. We solved Equation (1) using a fast iterative shrinkage-thresholding algorithm (FISTA) 4. Once each individual respiratory bin image is reconstructed, the most end-expiratory bin is used as a reference to estimate bin-to-bin 3D non-rigid deformation fields via image registration as described in reference 3. Finally, the registered bin images are combined to get the final image.
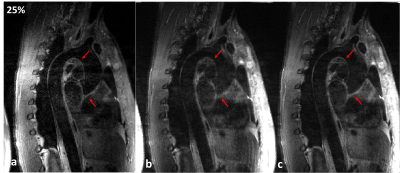
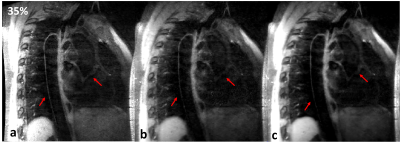
Experiments: Two healthy volunteers (male, age 25 and 28 years old) undergone thoracic MRI. The experimental data was acquired on two 3T MRI scanners during free-breathing (SIEMENS Tim Trio, Erlgen, Germany and United Imaging, shanghai, China). This study was approved by our institutional review board and written informed consent was obtained before experiment. For the proposed method, the phased array abdomen coil was used for signal reception. The relevant imaging parameters in the SIEMENS scanner were: TE/TR=20/600ms, trigger delay=60 ms, echo train length of imaging data was 37, sagittal acquisition with spectral-selective fat saturation, matrix size 256×198×56, voxel size 1.17×1.17×1.1 mm3, Average/Accelerated factor=2/1.4. The relevant imaging parameters in the united imaging scanner were: TE/TR=7.64/800ms, echo train length was 40, matrix 256 ×252×62 , Average=2, voxel size 1×1×1 mm3, a variable-density Cartesian sampling with accelerated factor of 3.5 was executed. For the traditional diaphragmatic navigator-gated acquisition, navigator accepted window on both scanners was 2.5mm, and the image acquisitive rates were 25% and 35% in the SIEMENS and United Imaging scanners, respectively.
Results
The average scan time with the proposed motion correction technique was about 6 minutes with high scan efficiency and no diaphragmatic navigator was gated. The average scan time for the navigator-gated and tracked acquisition was about 10 minutes with 40% scan efficiency. Therefore, the proposed acquisition scheme was approximately two times faster than the navigator-gated acquisition. The diagram of the data binning strategy and the motion corrected procedure required by the proposed technique is presented in Figure 2. The image results of Figure 3 and Figure 4 demonstrate that the proposed scheme can improve the vessel sharpness significantly by suppressing motion artifacts in image reconstruction. Additionally, the reconstructed volumes of the thoracic aorta by the proposed method show a comparable vessel length and sharpness to the navigator-gated reference acquisition.Conclusion
We proposed a new 3D thoracic aorta imaging scheme based on self-gating and affine respiratory motion correction technique. This scheme estimates the respiratory motion from the self-navigator data and corrects the motion in the reconstruction process. The results demonstrate that the proposed scheme yields image quality comparable to that of a 2.5 mm navigator-gated longer scan time acquisition, and also outperforms the non-corrected acquisition with the same acquisition time. Further evaluation of the proposed method in healthy volunteers and patients will be conducted in the future.Acknowledgements
This work was supported in part by the National Science Foundation of China (No.81901736, No.81571669, No.61471350, No.81729003, No. 61871373), Guangdong Provincial Key Laboratory of Medical Image Processing (No. 2017A050501026, No. 2018A0303130132). Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect those of the NSFC.References
1. Zhang Z, Fan Z, Carroll T J, et al. Three-dimensional T2-weighted MRI of the human femoral arterial vessel wall at 3.0 Tesla. Investigative radiology, 2009;44(9): 619.
2. Usman M, Ruijsink B, Nazir M S, et al. Free breathing whole-heart 3D CINE MRI with self-gated Cartesian trajectory. Magnetic resonance imaging, 2017; 38: 129-137.
3. Cruz G, Atkinson D, Henningsson M, et al. Highly efficient nonrigid motion‐corrected 3D whole‐heart coronary vessel wall imaging. Magnetic resonance in medicine, 2017; 77(5): 1894-1908.
4. Forman C, Piccini D, Grimm R, et al. Reduction of respiratory motion artifacts for free‐breathing whole‐heart coronary MRA by weighted iterative reconstruction. Magnetic resonance in medicine, 2015; 73(5): 1885-1895.
5. Zhang T, Cheng J Y, Potnick A G, et al. Fast pediatric 3D free‐breathing abdominal dynamic contrast enhanced MRI with high spatiotemporal resolution. Journal of Magnetic Resonance Imaging, 2015; 41(2): 460-473.
Figures