3341
Self-navigated abrupt Motion Correction for SNAP with 3D golden angle radial k-space sampling (GOAL-SNAP) in Carotid Artery T1 mapping1CBIR, Center for Biomedical Imaging Reserch, Tsinghua University, Beijing, China, 2School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
GOAL-SANP is a promising technique for carotid atherosclerotic plaque T1 mapping using 3D golden angle radial acquisition. However, sever patient abrupt motion is still a challenge for this sequence. In this study, a self-navigated retrospective motion correction scheme based on independent components analysis (ICA) and peak filtering was developed for GOAL-SNAP utilizing the advantage of its 3D golden angle radial trajectory. The results validated the feasibility of the proposed method to detect the abrupt motion (cough) and correct the related artifacts for carotid vessel wall imaging.
Introduction
The simultaneous noncontrast angiography and intraplaque hemorrhage imaging with 3D golden angle radial k-space sampling (GOAL-SNAP) has been proved to be a promising noninvasive T1 mapping technique for carotid atherosclerotic plaque [1]. Although the golden angel radial k-space sampling is not sensitive to mild motion, severe abrupt motion, such as cough, is still a challenge for carotid vessel wall imaging. In the golden angle radial sampling, k-space center is acquired again and again, which can be used as a navigator to detect the abrupt motion and thus further correct the related motion artifacts. Thus, this study proposed a self-navigated retrospective abrupt motion correction scheme for GOAL-SNAP sequence in carotid vessel wall T1 mapping.Methods
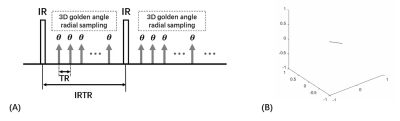
GOAL-SNAP sequenceGOAL-SANP sequence consists of an inversion recovery (IR) prepulse and a series of 3D golden angle radial acquisition [1] (Figure 1A). Its acquisition trajectory is shown in Figure 1B.
Data acquisition
After institutional review board approval, six healthy volunteers (3 males, 24.3 ± 2.5 y) were imaged on a 3T MR scanner (Ingenia CX, Philips Healthcare, The Netherlands) with a 32-channel head coil jointed with an 8-channel carotid coil, using GOAL-SNAP sequence, with the following scan parameters: FOV = 100x100x100mm3, spatial resolution = 0.8x0.8x0.8 mm3, TR/TE = 11.3 ms/4.1 ms, IRTR = 2000 ms, TFE factor = 155, density of radial acquisition = 200%, scan duration = 6 min 40 s. All the volunteers were asked to cough 3 to 5 times during the first 100% radial acquisitions and to keep free-breathing for the rest 100%. A bellow was put on the neck to recode the acute cough motion as the reference for abrupt motion.
Self-navigated Motion Correction
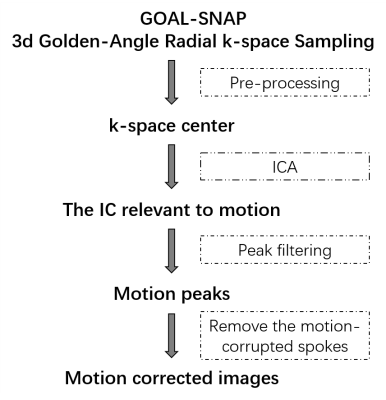
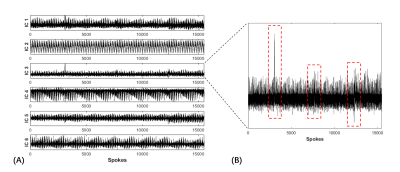
In this study, the abrupt motion was detected based on independent components analysis (ICA) [2]. Then a filtering algorithm was applied to automatically detect the abrupt motion. The flowchart of retrospective motion correction, including motion extraction from the k-space center, motion detection, and motion correction, is shown in Figure 2. After coil compression, ICA was performed on the k-space center amplitudes which were extracted from the center point of each radial line to obtain independent components (ICs) with a fixed number at six [3-4]. The IC with abnormal peaks and oscillations was empirically selected as the motion component. To better select the motion corrupted data caused by abrupt motion, a motion peak filtering algorithm [5] was implemented, including three steps: (1) Calculate the product of the average and standard deviation (STD) of the area centered on the current point with the window size set as TFE factor (=155). (2) Calculate the STD of 155 points on the left and right sides of the former area, 310 points in total. (3) The ratio of (1) to (2) was considered as the estimation of coughing motion signals. Main peaks corresponding to coughing motion were then detected using the findpeaks function in MATLAB. Then, the spokes acquired within two seconds before and five seconds after are considered as motion-corrupted. The corresponding spokes were removed and replaced with the free-motion spokes acquired during the second 100% radial acquisition to correct the motion artifacts (motion-corrected k-space dataset).
Reconstruction, T1 mapping and Validation test
Motion-corrected images were reconstructed using the motion-corrected k-space dataset and compared with the motion-corrupted images which were reconstructed using the first 100% radial acquisitions with cough. Sliding windowing reconstruction method [1] was used with temporal window width = 19. Low-rank modeling and sparsity constraints method [6] was applied to further reduce the undersampling artifacts. Corresponding T1 mapping was estimated by fitting the reconstructed motion-corrected and mition–corrupted images to the theoretically simulated signal, respectively [1].
Results
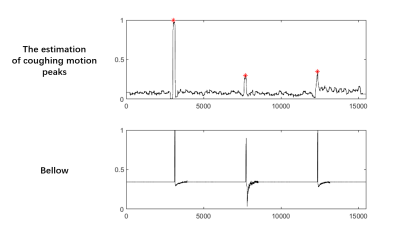
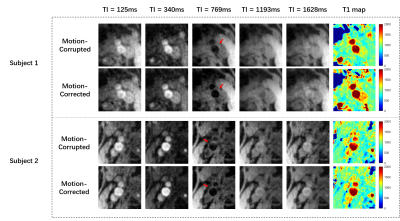
A total of 25 coughs, within one second according to the indication of the bellow, were correctly detected by the proposed algorithm (25/25, accuracy: 100%). An example of the extracted ICs and the motion corresponded IC is shown in Figures 3A and B. The motion peaks of one subject detected by the proposed algorithm with the reference of bellow are shown in Figure 4. As we can see, the cough detected corresponds well to the record of bellow. Figure 5 demonstrates the reconstructed GOAL-SNAP images at multiple inversion times (TI) and the corresponding T1 map from two subjects. Compared to the motion-corrupted images, the degree of motion artifacts was reduced after correction with the proposed method. The carotid vessel walls are much clearer than before (Figure 4, TI = 769ms), indicating the improvement of the image quality. The corresponding estimated T1 maps are shown in the last column.Discussion and Conclusion
In this work, a motion correction pipeline for GOAL-SNAP in carotid artery T1 mapping was described. This work focus on the correction of abrupt motion, however, patients’ involuntary motion may be another challenge that needs to be overcome. Moreover, quantitative assessments of the improvement of image quality due to the proposed algorithm should be investigated in future works. In conclusion, a self-navigated retrospective abrupt motion correction scheme has been developed in this work for GOAL-SNAP sequence in carotid vessel wall T1 mapping. Its feasibility to detect the abrupt motion (cough) and correct the related artifacts for carotid vessel wall imaging was validated.Acknowledgements
References
[1] Qi H , Sun J , Qiao H , et al. Carotid Intraplaque Hemorrhage Imaging with Quantitative Vessel Wall T1 Mapping: Technical Development and Initial Experience[J]. Radiology, 2017, 287(1):170526.
[2] Hyvärinen A, Oja E. Independent component analysis: algorithms and applications[J]. Neural networks, 2000, 13(4-5): 411-430.
[3] Bonanno G , Hays A G , Weiss R G , et al. Self-gated golden angle spiral cine MRI for coronary endothelial function assessment[J]. Magnetic Resonance in Medicine.
[4] Free‐running 3D whole heart myocardial T1 mapping with isotropic spatial resolution[J]. Magnetic Resonance in Medicine, 2019, 82(4).
[5] Carroll, T. J, et al. Automatic calculation of the arterial input function for cerebral perfusion imaging with MR imaging[J]. Radiology, 2003, 227(2): 593-600.
[6] Haikun Qi, et al. Highly undersampled kooshball reconstruction with low-rank modeling and sparsity constraints for high-resolution T1 Mapping. [C]//Proceedings of the 26th Annual Meeting of ISMRM, Singapore. 2018.
Figures