3339
Motion Correction of T2SE Multi-Pass Acquisitions for Super-Resolution in the Slice Direction
Eric A. Borisch1, Soudabeh Kargar1, Akira Kawashima2, and Stephen J. Riederer1
1Radiology, Mayo Clinic, Rochester, MN, United States, 2Radiology, Mayo Clinic, Phoenix, AZ, United States
1Radiology, Mayo Clinic, Rochester, MN, United States, 2Radiology, Mayo Clinic, Phoenix, AZ, United States
Synopsis
A new method for retrospectively correcting subtle transverse slice-to-slice motion in a set of overlapped axial slices is introduced. Such motion can be problematic for high through-plane resolution imaging of the pelvis due to peristalsis of the rectum. The method exploits the known slice-to-slice correlation to determine offsets as well as characteristics of the multi-pass acquisition. When applied to 16 T2-weighted fast spin-echo exams of the prostate the method shows significant improvement in prostate-rectum margin clarity and overall image quality as assessed on sagittal reformats from axial images.
Introduction
T2-weighted fast spin-echo (T2 FSE) multi-slice imaging typically has limited through-plane resolution, generally 3-8x coarser than inplane. Although 3D spin-echo methods (e.g. CUBE, SPACE, VISTA) attempt to address this, they still suffer from inferior resolution and contrast vs. 2D. Super resolution using overlapped slices [1, 2] is an approach for providing improved through-plane resolution. The acquisition contains multiple passes of a multi-slice sequence, non-overlapping within each pass, but the combination of all passes produces the desired overlapped sampling. Subsequent compensation of the slice profile provides resolution improvement, allowing high resolution reformats, e.g. sagittal reformats from axial acquisition. This can work effectively for relatively stationary imaging volumes, such as the brain. However, in regions subject to motion, even slight displacements of the object between passes can cause an objectionable “stair-step” artifact. The motion correction in this work leverages characteristics of the acquisition to improve the diagnostic quality of the resulting images.Methods
When slices are acquired with overlap (e.g. 3mm-thick slices with 1mm steps have 2mm overlap), a correlation can be expected between adjacent slices. With two images that are significantly correlated, a small translational shift occurring between the images can be determined via their two-dimensional cross correlation. The cross correlation $$$R_{MN}$$$ between images $$$M$$$ and $$$N$$$ can be efficiently calculated via 2D Fourier transformations: $$R_{MN}=\mathscr{F}^{-1} \left\{\mathscr{F}\left(M^-\right)\circ\mathscr{F}\left(N\right)\right\}$$ With $$$\circ$$$ indicating element-wise multiplication, and $$$M^-$$$ a flipped version of $$$M$$$ with reversed $$$x$$$ and $$$y$$$ axes, producing a cross-correlation rather than a convolution. Locating the peak within $$$R_{MN}(x,y)$$$ of the two highly correlated images provides the sub-pixel $$$(x,y)$$$ shift which maximizes their correlation (alignment).For axial prostate imaging centered on the prostate, motion of the prostate due to peristalsis of the rectum is to be expected, while regions such as bone remain stationary. To focus on the prostate, the central 20% of the each image (here a 4.8 x 4.8cm patch) forms the input for cross-correlations, and detected slice-to-slice shifts are recorded for each pair of adjacent slices. Sample results are shown in Figure 1.
Directly applying corrections for the detected shifts would have the effect of trying to "straighten" out the features of the body in the through-plane direction. Existing [3, 4] approaches using cross correlation process a time-series, where this is not an issue. However, consider the acquisition of a tilted (relative to slice direction) cylinder; each slice would have a detected shift that tries to undesirably straighten the stack, misrepresenting ground truth. Additionally knowledge of the acquisition ordering can be used to constrain the final motion-compensating adjustments. In this acquisition, 78 slices are acquired in six passes to avoid interference. Slices 1, 7, 13, ... , 73 are acquired within the same pass, and any motion experienced by one is likely common to all. In practice, this effect manifests in transverse reformats as repetitive undulations or "stair-stepping" with a period of six slices.
By filtering the detected offsets along the slice direction for only those features we are most interested in — the harmonics of a period of 6 in this case — we can improve stability while simultaneously rejecting the straightening tendency described above, which manifest as low-frequency components. By applying (via FFTs and phase-shifts) the filtered offsets (Figure 2) in the opposite direction between each pair of consecutive slices, the motion corruption can be effectively suppressed.
The motion correction method was applied to T2 FSE axial imaging of the prostate. Under an IRB-approved protocol and after written consent, 16 subjects were imaged. For each subject 78 3mm thick slices were acquired in six passes with 2 mm slice-to-slice overlap. After the above-described motion correction, the slices were processed [2] to yield the targeted nominal 1 mm thick axial slices. Sagittal reformats were generated both with and without motion correction. A radiologist with over 10 years of experience in prostate MR scored each pair of (randomized presentation) sagittal reformats for diagnostic quality (Non-diagnostic/Marginal/Diagnostic) and the clarity of the rectal-prostatic margin.
Results
Motion-corrected results were significantly preferred for both diagnostic quality and rectal-prostatic margin clarity (Figure 3), with respective Wilcoxon Signed-Rank scores of $$$p=0.012$$$ and $$$p=0.001$$$. Examples of moderate and strong (Figure 4) preference for motion correction are shown.Discussion
A purely post-processing approach to motion correction for overlapped acquisitions was designed and implemented; the initial radiological evaluation was very favorable. This technique leverages a property of overlapped acquisitions, built-in correlation between slices, and utilizes knowledge of the acquisition specifics to improve the alignment results. Current limitations of the work include introducing artifact outside the organ of interest, as each slice is aligned as a whole. As this is a post processing technique, the original images are available, such that the diagnostic impact of this is reduced. Future work may consider a modified non-rigid correction by modulating the applied correction further from the region of interest. The final goal is to reduce the number of scans required by providing near-native sagittal (via reformatting; Figure 5) performance from the axial acquisition.Acknowledgements
NIH RR018898, Mayo Discovery-Translation ProgramReferences
- Greenspan, et al. MRI inter-slice reconstruction using super-resolution, Magnetic Resonance Imaging, 2002; 20:437-446 https://doi.org/10.1016/S0730-725X(02)00511-8
- Kargar, S., et al. Use of kZ‐space for high through‐plane resolution in multislice MRI: Application to prostate. Magn Reson Med. 2019; 81: 3691– 3704. https://doi.org/10.1002/mrm.27691
- Gupta, S. N., et al. Fast method for correcting image misregistration due to organ motion in time‐series MRI data. Magn. Reson. Med. 2003, 49: 506-514. https://doi.org/10.1002/mrm.10394
- Gao, B., et al. Estimation of cardiac motion in cine-MRI sequences by correlation transform optical flow of monogenic features distance. Phys. Med. Biol. 2016 61 8640 https://doi.org/10.1088/1361-6560/61/24/8640
Figures
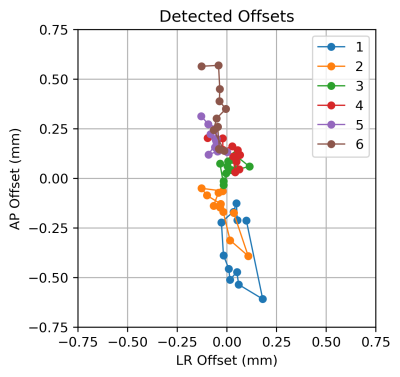
Figure 1: Final image offsets (in mm; A/P vertically, L/R horizontally). The 78 slices analyzed are colored by pass number. The plotted image offsets are formed via the integration of the resolved slice-to-slice shifts. This case is also displayed in Figure 2.
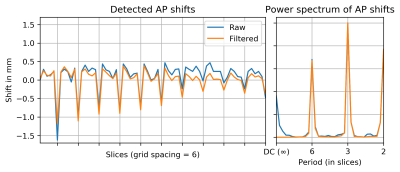
Figure 2: Detected Anterior-Posterior slice-to-slice shifts (left) and power spectrum (right) with and without filtering. (Left-Right shifts were significantly smaller and not shown; see Figure 1.)
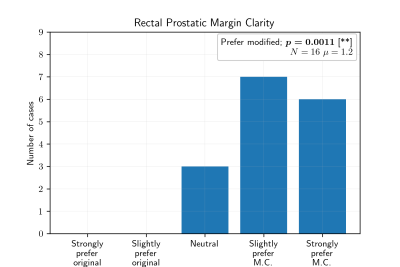
Figure 3: Scores for rectal prostatic margin clarity.
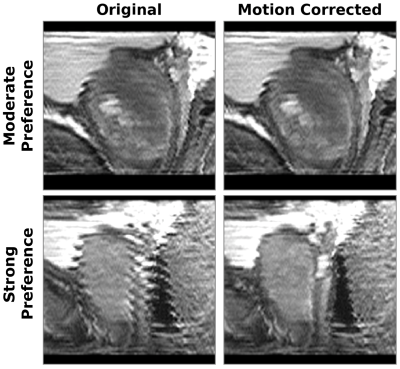
Figure 4: Example of moderate (top) and strong (bottom) preference for motion correction; sagittal reformatted original (left) and motion corrected (right).
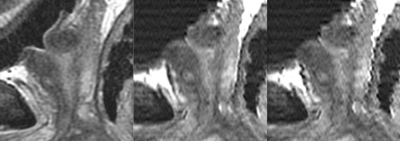
Figure 5: Native sagittal T2SE acquisition (left) in comparison to (acquired later) reformatted axial-source images with (center) and without (right) motion correction.