3332
Actuator-Free MR Elastography of the Liver: Imaging Liver Tumors with Viscoelastic Intrinsic MRE1Génie mécanique, Université de Sherbrooke, Sherbrooke, QC, Canada, 2MR Clinical Science, Philips Healthcare, Markham, ON, Canada, 3Département de radiologie, Université de Montréal, Montréal, QC, Canada
Synopsis
A new intrinsic activation MR Elastography method is presented through a proof of concept for imaging the viscoelasticity of a liver tumor. Results are presented for a healthy volunteer and a focal nodular hyperplasia subject. Relative stiffness, damping and compressibility maps clearly indicate the location of the tumor and are in agreement with mechanical property values previously reported in the literature. The method shows promise for the diagnosis of focal liver lesions and provides insight into new physiologically related biomarkers for these lesions.
Introduction:
Unlike traditional extrinsic MR Elastography (MRE), intrinsic MRE (iMRE) does not require an external vibration source to generate the deformation field used for viscoelastic characterization. This simplifies data acquisition and provides elasticity information at physiologically relevant frequencies (≈1 Hz). Intrinsic activation MRE has been previously demonstrated in the brain1, and the liver2-4, however hepatic iMRE results to date have been limited to strain analysis of MR tagging displacement data. Here, Non-Linear Inversion (NLI) based iMRE of the liver is presented via relative viscoelastic property distributions within a healthy subject and a subject with focal nodular hyperplasia (FNH).Methods:
Full 3D displacement field data was acquired from a healthy volunteer and a FNH subject on a clinical 3T system (Ingenia, Philips Healthcare) using a 4D quantitative flow sequence (Q–Flow), similar to Gordon-Wylie et al.5. Retrospective gating was used to synchronize eight phases within the cardiac cycle in conjunction with a navigator-gated respiration state or during end-expiration breath-hold. These motions were then treated with the subzone based NLI-MRE reconstruction6. As has been reported recently, viscoelastic property reconstruction at physiological frequencies is non-unique to a scalar multiplier7. Viscoelastic property reconstructions were performed to obtain the spatial distribution of the shear storage modulus, or real shear modulus, $$$G^\prime$$$, and the shear loss modulus, or imaginary shear modulus, $$$G^{\prime\prime}$$$. The resulting property maps were normalized by the mean property value to investigate the relative property distribution within liver, including the focal lesion of the tumor. The viscoelastic shear damping-ratio distribution (DR) was generated from the reconstructed storage and loss modulus distributions, $$$DR = \frac{G^{\prime\prime}}{2G^\prime}$$$, with results shown as relative distributions. In addition to the typical viscoelastic tissue properties, the bulk modulus distribution within the tissue was also reconstructed8, to reflect the effective compressibility of soft tissues at low frequencies9.Results:
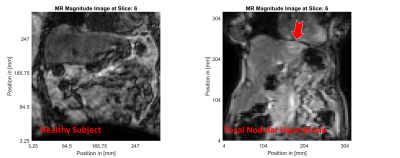
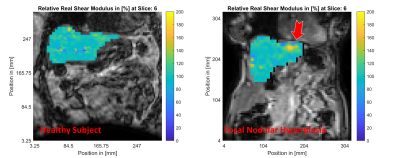
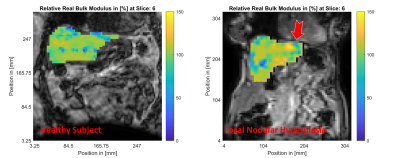
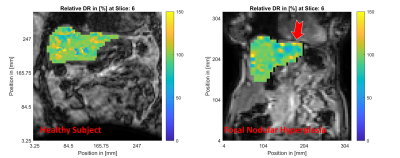
MR magnitude images of similar slices within the healthy (left column) and FNH (right column) subjects are shown in Figure 1. The location of the focal lesion in the left liver lobe is indicated by the red arrow. The normalized shear storage modulus ($$$G^\prime$$$) distributions within the healthy and FNH subjects are shown in Figure 2. Figure 3 shows the corresponding relative bulk modulus distributions within the two subjects, while Figure 4 shows the distributions of the relative damping ratio (DR).The focal lesion is well localized in all three iMRE images, with storage modulus variations of over 200% and bulk modulus variations of over 150%. Damping ratio is reduced by roughly 50% within the region of the tumor.
Discussion:
The storage modulus increase of over 200% observed within the FNH tumor compared to healthy tissue are similar to the stiffness changes observed in FNH tumors by Hennedige et al. using 60 Hz external activation MRE10. This result is consistent with a structurally viscoelastic description of the liver, where the relative shear modulus of the healthy tissue and the tumor do not change significantly with frequency (even if the absolute values are frequency dependent).In terms of tissue attenuation, we observe a decrease of roughly 50% in the damping ratio of the tumor relative to the healthy tissue, which is in contrast to the elevated damping levels observed by Garteiser et al. using 50 Hz external activation MRE11. These results indicate different modes of frequency dependence for the healthy tissue and the tumor, which may be a useful biomarker to investigate for MRE diagnosis of focal liver lesions.
In addition to significant changes in the shear stiffness of the tumor relative to the surrounding healthy tissue, the local compressibility of the tumor, as measured by the bulk modulus, increased by over 150% compared to its surroundings. At the low frequencies used in iMRE, these changes in bulk modulus may reflect either a change in the hydraulic conductivity of these circumscribed tumors, or a significant change in their structural rigidity, both of which are interesting biomarkers for further study.
Conclusions:
Low frequency iMRE offers an exciting new option for the diagnosis of focal liver lesions. Based on a relatively simple imaging protocol, the method provides 3D, full-volume images of the viscoelastic properties of the liver. The use of the intrinsic cardiac cycle for mechanical excitation eliminates the need for an external vibration source, and thus the possibility that a poorly placed actuation pad renders the MRE imaging exam clinically unusable. In addition, iMRE offers the potential for interesting new biomarkers for liver tumors, such as their damping behavior and compressibility, whose physiological sources merit further study.Acknowledgements
Quebec Bio-imaging Network QBIN 13.3 awarded to Elijah Van Houten
Fonds de Recherche du Québec en Santé and Fondation de l'Association des Radiologistes du Québec (FRQS-ARQ #34939) awarded to An Tang.
References
1. Weaver, John B, et al. "Brain mechanical property measurement using MRE with intrinsic activation." Physics in Medicine & Biology 57.22 (2012): 7275.
2. Chung, Sohae, et al. "Liver stiffness assessment with tagged MRI of cardiac‐induced liver motion in cirrhosis patients." Journal of Magnetic Resonance Imaging 39.5 (2014): 1301-1307.
3. Lefebvre, Thierry, et al. "MRI cine‐tagging of cardiac‐induced motion for noninvasive staging of liver fibrosis." Journal of Magnetic Resonance Imaging (2019).
4. Giammarinaro, Bruno, et al. "Passive elastography monitoring of a high intensity ultrasound treatment: A study of feasibility in in vitro liver." The Journal of the Acoustical Society of America 144.3 (2018): 1925-1925.
5. Gordon-Wylie, Scott W, et al. "MR elastography at 1 Hz of gelatin phantoms using 3D or 4D acquisition." Journal of Magnetic Resonance 296 (2018): 112-120.
6. Tan, Likun, et al. "Gradient-based optimization for poroelastic and viscoelastic MR elastography." IEEE transactions on medical imaging 36.1 (2016): 236-250.
7. McGarry, Matthew, et al. "Uniqueness of poroelastic and viscoelastic nonlinear inversion MR elastography at low frequencies." Physics in Medicine & Biology 64.7 (2019): 075006.
8. Van Houten, Elijah EW, et al. "A three-parameter mechanical property reconstruction method for MR-based elastic property imaging." IEEE transactions on medical imaging 24.3 (2005): 311-324.
9. McGarry, M. D. J., et al. "Suitability of poroelastic and viscoelastic mechanical models for high and low frequency MR elastography." Medical physics 42.2 (2015): 947-957.
10. Hennedige, Tiffany P., et al. "Comparison of magnetic resonance elastography and diffusion-weighted imaging for differentiating benign and malignant liver lesions." European radiology 26.2 (2016): 398-406.
11. Garteiser, Philippe, et al. "MR elastography of liver tumours: value of viscoelastic properties for tumour characterisation." European radiology 22.10 (2012): 2169-2177.
Figures