3331
In Vivo Cardiac MR Elastography using Simultaneous Multi Slice Imaging1Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 2Philips Healthcare, Guildford, United Kingdom, 3Inserm U1148, University Paris, Paris, France, 4Departments of Biomedical Engineering and Cardiac Surgery, University of Michigan, Ann Arbor, Ann Arbor, United Kingdom
Synopsis
MR Elastography is a technique to evaluate the biomechanical properties of soft tissues noninvasively. Its application in the heart is very challenging due to several factors including actuator hardware, robust MR sequences, and suitable reconstruction strategies. One of the biggest limitations for data acquisition of cardiac MRE scans is the breath hold duration which is up to 21 heart beats, repeated several times for a full dataset. The main goal of this study was the implementation of an MR sequence with simultaneous multi slice acquisition capability to reduce the breath hold duration and increase comfort and compliance for the subjects.
INTRODUCTION
MR Elastography (MRE) is a technique to evaluate the biomechanical properties of soft tissues noninvasively and in vivo1. These biomechanical properties often change with diseases, making MRE a valuable tool for diagnosis and staging of disease. It is very challenging to apply this technology in the heart due to several factors including actuator hardware, robust MR sequences, and reconstruction strategies suitable for reconstructing the long wavelengths observed in the myocardium. One of the biggest limitations for data acquisition of cardiac MRE scans is the breath hold duration of the MR sequence which is up to 21 heart beats, repeated several times for a full dataset. This breath hold duration combined with the vibrations induced by the continuously running transducer to ensure the steady state periodic behaviour of the waves does not make it very comfortable for the subjects. Simultaneous Multi Slice (SMS)2 imaging has the potential to reduce scan time by the number of simultaneously excited slices. The main goal of this study was the implementation and testing of an MR sequence with simultaneous multi slice acquisition capability for cardiac MRE in order to reduce the breath hold duration and thus providing comfort and compliance for the subjects. Cardiac MRE data were acquired using a gravitational transducer3 in combination with a single-shot SE-EPI-MRE sequence employing inner volume excitation4 in combination with SMS acquisitions.METHODS
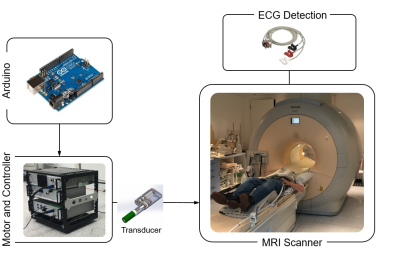
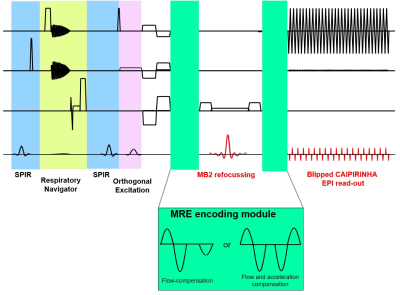
Hardware: The MRE gravitational transducer concept5 (Fig.1) provided shear waves for cardiac MRE with the transducer running uninterruptedly to maintain the periodic steady-state of the waves. The synchronization of the hardware with the MRE sequence has been provided by measuring the exact ECG detection time point in the pulse sequence and delaying the excitation accordingly. The correct position of the transducer was ensured by sending periodic triggers with an Arduino MEGA.MR Sequence: A cardiac-triggered single-shot SE-EPI MRE sequence combined with inner-volume excitation and SMS imaging was implemented as shown in Fig.2. The MRE motion encoding gradients (MEGs) can be either only flow compensated or flow and acceleration compensated. The sequence provides increasing delays to achieve different mechanical wave phases in addition to the delay used to synchronise the sequence with the transducer.
Data acquisition: Measurements were performed on a 3T Achieva MR scanner (Philips Healthcare, The Netherlands) in breath-hold with imaging parameters: TR=3RR; TE = 47ms/60ms without SMS imaging depending on the motion compensation scheme of the MEGs and TR=2RR and TE=87ms with SMS imaging for a 2x2x4mm3 voxel; SENSE factor = 2 without SMS imaging; MB factor = 2; ETL = 41/83. 3 and 4 slices were acquired without SMS and with SMS imaging, respectively. Four healthy volunteers (3 females) were scanned with the transducer placed centrally near the sternum of the subject under the anterior part of a 32-channel cardiac coil. MEGs were applied in slice selection, phase encoding, and readout directions including a reference scan without MEGs. MRE vibration frequency was 80Hz.
Data preprocessing: Complex MRE images were denoised using the BM4D algorithm6 and unwrapped for each MEG direction separately with FSL prelude7. To remove residual motion, all magnitude images were slice-wise co-registered to the first frame using elastix8 and corresponding non-rigid transformation fields were applied to the unwrapped phase images.
Reconstruction: From the complex displacement fields, the time point in the period where the maximum displacement occurs is found, for every voxel. Using the difference in these time point values between neighbouring pixels, along with the pixel dimensions, wave speeds and therefore elasticity values can be calculated and are averaged over all slices.
RESULTS AND DISCUSSION
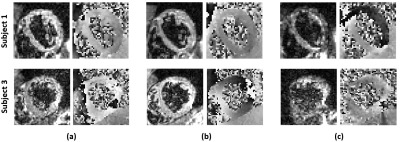
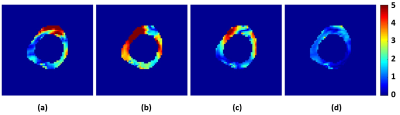
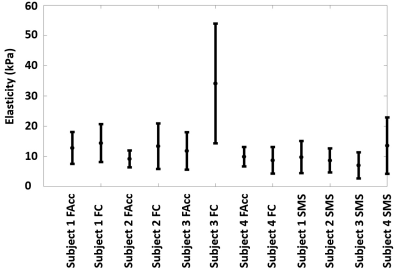
Fig. 3 shows the magnitude and phase images for different scans to demonstrate data quality from two subjects. Magnitude images show complete blood suppression and clear delineation of the myocardium although some noise can be observed in the SMS images. Quality of phase images is more similar between SMS and non-SMS methods but varies between subjects. Fig. 4 illustrates the magnitude of the complex displacement fields from different measurement directions. Fig.5 displays the reconstructed elasticity results averaged over all slices for all 4 subjects and all three sequences. There is general agreement within and between subjects with all techniques. However, misalignment of the unwrapped phase images as seen in subject 3 (Fig. 3) can cause erroneous elasticity estimation.CONCLUSION
We have shown here that SMS and inner-volume imaging can be successfully employed in cardiac MRE to reduce breath-hold time by a third while simultaneously increasing slice coverage with acceptable compromise in data quality and elasticity estimation. Furthermore, flow-only compensating MEGs are sufficient to suppress artefacts while keeping TE short without introducing extra biases in estimated elasticity. Correct phase unwrapping remains the biggest challenge of this method and need to be addressed more robustly in future work.Acknowledgements
This work was supported by the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z] and the EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1, EP/N011554/1 and EP/R0037866/1).References
1) Glaser KJ et al. Review of MR elastography applications and recent developments. J Magn Reson Imaging. 2012; 36:757-774.
2) Setsompop K et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magnetic Resonance in Medicine. 2012; 67(5):1210-1224.
3) Runge JR et al. A Novel MR Elastography Transducer Concept Based on a Rotational Eccentric Mass: Preliminary Experiences with the Gravitational Transducer. Phys. Med. Biol. 2019; 64.
4) Feinberg DA et al. Inner Volume MR Imaging: Technical Concepts and Their Application. Radiology. 1985; 156:743-747.
5) Dokumaci AS et al. In Vivo Cardiac MR Elastography with a Gravitational Transducer. In: Intl. Soc. Mag. Reson. Med. 27. Vol 3964. Montreal; 2019.
6) Maggioni M et al. A Nonlocal Transform-Domain Filter for Volumetric Data Denoising and Reconstruction. IEEE Trans. Image Process. 2013; 22:119-133.
7) Jenkinson M et al. Fast, automated, N‐dimensional phase‐unwrapping algorithm. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2003; 49.1:193-197.
8) Klein S et al. elastix: a toolbox for intensity based medical image registration. IEEE Transactions on Medical Imaging, 2010; 29:196-205.
Figures