3329
In Vivo Aortic MR Elastography using Free-Breathing and Single-Breath-Hold SE-EPI: Inter-Scanner Reproducibility at 1.5T and 3T1Department of Radiology, The Ohio State University Wexner Medical Center, Columbus, OH, United States, 2Department of Biomedical Engineering, The Ohio State University, Columbus, OH, United States, 3Internal Medicine-Division of Cardiology, The Ohio State University Wexner Medical Center, Columbus, OH, United States
Synopsis
In vivo MR elastography (MRE) allows non-invasive estimation of aortic stiffness. Recently, the feasibility of multi-slice free-breathing (FB) spin-echo echo-planar imaging (SE-EPI) aortic MRE was investigated. In this study, a single breath-hold (SBH) SE-EPI aortic MRE protocol was proposed and compared to FB SE-EPI aortic MRE. Moreover, in vivo FB and SBH SE-EPI aortic MRE were studied using both1.5T and 3T scanners. The main aim was to assess inter-scanner reproducibilities of FB and SBH SE-EPI aortic MRE.
Introduction
Recent development in aortic MR elastography (MRE) has provided the opportunity to non-invasively study in vivo aortic stiffness in the healthy aorta [1-3] as well as in the aorta with abnormalities [4-6]. Aortic stiffness can be a potential biomarker for multiple cardiovascular diseases, such as systemic arterial hypertension (SAH) [7] and abdominal aortic aneurysm (AAA) [8].Recently, the feasibility of multi-slice free-breathing (FB) SE-EPI aortic MRE and its comparison to the established GRE aortic MRE was investigated at 3T [9]. In the present study, a single breath-hold (SBH) SE-EPI aortic MRE protocol was proposed to acquire multi-slice and multi-directional in vivo aortic MRE data, reducing the potential measurement errors caused by prolonged scan time and inconsistent breath-holds.
Currently, the performance of FB and SBH SE-EPI aortic MRE on a 1.5T scanner remains unknown. Compared to 3T scanners, 1.5T scanners are more commonly employed for cardiovascular imaging. However, signal-to-noise ratio (SNR) is reduced due to lower field strength when similar imaging parameters are applied, which can potentially bias the stiffness estimates.
Therefore, the main goal of this work is to study the inter-scanner reproducibilities of in vivo SE-EPI aortic MRE when using (1) a FB protocol and (2) a SBH protocol.
Methods
A cardiac-gated SE-EPI MRE sequence was implemented. Prospective gating was used to choose the appropriate trigger delay for avoiding flow- or cardiac motion-induced artifacts by acquiring data during diastole. The external mechanical vibration was synchronized to the cardiac gating signal and triggered immediately after detecting the gating signal for the first MRE phase offset. The number of vibration cycles was calculated based on the total duration of the selected trigger delay and TR. The other MRE phase offsets were triggered by shifting the starting time of the external mechanical vibration accordingly.To further reduce the effect of aortic flow on MRE phase images, the motion-encoding gradient (MEG) was designed to be both zero- and first-moment-nulled. Moreover, the first moment of the readout gradient was nulled for the center k-space line. EPI ghosting and distortion were effectively corrected via a non-phase-encoded reference scan and protocol optimization. To further reduce the undesired signal that can potentially cause ghosts, a saturation band was used to suppress the unwanted signal from the anterior surface of the subject (i.e., eliminating signals from the anterior abdomen and chest). Chemical shift and off-resonance artifacts were reduced through gradient reversal technique [10].
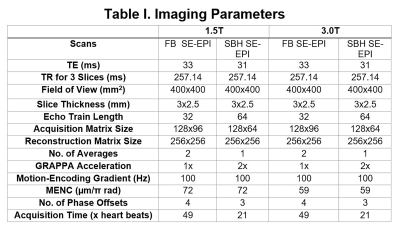
In this ongoing study, 4 healthy volunteers (age: 33±9.26 years) were recruited. MR imaging was performed on a 1.5T (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) and 3T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) MR scanners using the developed cardiac-gated SE-EPI MRE sequence. Imaging parameters for FB and SBH protocols are summarized in Table I. TR was selected to accommodate three slices.
In the first scan, both FB and SBH scans were performed during the same sitting. To determine the inter-scanner reproducibility of SE-EPI aortic MRE, each volunteer was also scanned on a different scanner at a different site after the first scan. Aortic MRE data were processed using MRElab (Mayo Clinic, Rochester, MN). Eight 4th-order Butterworth band-pass directional filters with cutoff of 1-40waves/FOV were used to eliminate the undesirable noise, longitudinal and reflected waves. Subsequently, 3D Local-Frequency Estimation (LFE) inversion was performed to obtain the weighted effective stiffness map from each motion-encoding direction [11]. The weighing was based on the first-harmonic amplitude from each motion-encoding direction.
Data are presented as mean ± standard deviation. Bland-Altman analysis was performed to assess inter-scanner reproducibility.
Results and Discussion
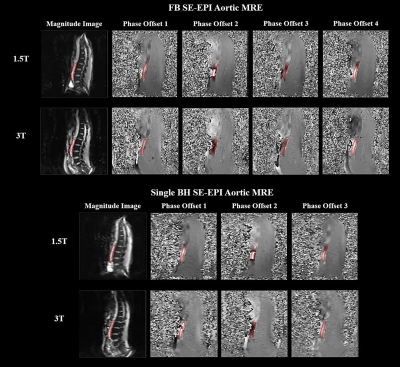
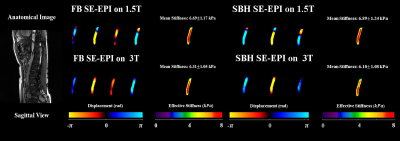
No significant artifacts were observed in FB or SBH SE-EPI MRE. Figure 1 demonstrates the magnitude images and raw MRE phase offsets.Discernible waves were observed in both FB and SBH SE-EPI scans. Figure 2 displays the anatomical image, snapshots of propagating waves within the aorta and the corresponding stiffness maps measured using two different scanners. Similar aortic stiffness maps were observed in all cases. The reported aortic stiffness is the average stiffness across 3 slices.
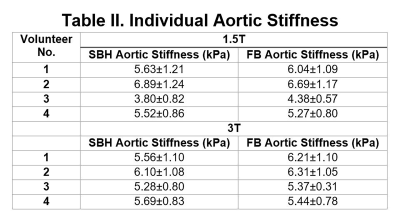
Table II shows the effective aortic stiffness for individual volunteers. FB and SBH SE-EPI aortic MRE yielded similar stiffness measures except in one volunteer (i.e., volunteer 3) who experienced variable heart rates and high flow during the second scan.
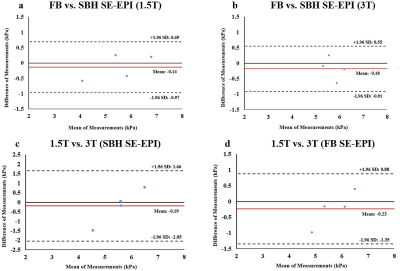
FB and SBH SE-EPI aortic MRE are reproducible on both scanners except for volunteer 3. Figure 3(a-d) demonstrate Bland–Altman plots.
Conclusion
The inter-scanner reproducibilities of FB and SBH SE-EPI aortic MRE were assessed in 4 volunteers at 1.5T and 3T. FB SE-EPI aortic MRE can potentially be a good alternative for patients who have challenges in holding their breath. To comprehensively assess the reproducibilities of FB and SBH SE-EPI aortic MRE on both 1.5T and 3T scanners, further investigation is needed by recruiting additional volunteers.Acknowledgements
The authors acknowledge grant sponsor: NIH–NHLBI (grant number: NIH-R01HL124096).References
1. Damughatla AR, Raterman B, Sharkey-Toppen T, et al. Quantification of aortic stiffness using MR Elastography and its comparison to MRI-based pulse wave velocity. J Magn Reson Imaging. 2015;41(1):44-51. doi:10.1002/jmri.24506
2. Kenyhercz WE, Raterman B, Illapani VSP, et al. Quantification of aortic stiffness using magnetic resonance elastography: Measurement reproducibility, pulse wave velocity comparison, changes over cardiac cycle, and relationship with age. Magn Reson Med. 2016;75(5):1920-1926.
3. Woodrum DA, Romano AJ, Lerman A, et al. Vascular wall elasticity measurement by magnetic resonance imaging. Magn Reson Med. 2006;56(3):593-600. doi:10.1002/mrm.20991
4. Dong H, Mazumder R, Illapani VSP, Mo X, White RD, Kolipaka A. In vivo quantification of aortic stiffness using MR elastography in hypertensive porcine model. Magn Reson Med. 2017;78(6):2315-2321.
5. Kolipaka A, Illapani VSP, Kenyhercz W, et al. Quantification of abdominal aortic aneurysm stiffness using magnetic resonance elastography and its comparison to aneurysm diameter. J Vasc Surg. 2016;64(4):966-974.
6. Kolipaka A, Woodrum D, Araoz PA, Ehman RL. MR elastography of the in vivo abdominal aorta: A feasibility study for comparing aortic stiffness between hypertensives and normotensives. J Magn Reson Imaging. 2012;35(3):582-586.
7. Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: Emerging concepts. Hypertension. 2010. doi:10.1161/HYPERTENSIONAHA.107.090464
8. Vorp DA, Vande Geest JP. Biomechanical determinants of abdominal aortic aneurysm rupture. Arterioscler Thromb Vasc Biol. 2005;25(8):1558-1566.
9. Dong H, Jin N, Kalra P, White R, Kolipaka A. In Vivo Quantification of Aortic Stiffness using a Multi-Slice Spin-Echo Echo-Planar Imaging Sequence: A Comparison to a Gradient-Recalled Echo Sequence. In: 27th Scientific Meeting of International Society of Magnetic Resonance in Medicine (ISMRM). Montreal, QC; 2019.
10. Nagy Z, Weiskopf N. Efficient fat suppression by slice-selection gradient reversal in twice-refocused diffusion encoding. Magn Reson Med. 2008. doi:10.1002/mrm.21746
11. Knutsson H, Westin CF, Granlund G. Local multiscale frequency and bandwidth estimation. In: 1st International Conference on Image Processing. 1994: 36–40.
Figures