3327
Free Breathing Magnetic Resonance Elastography in Patients with Idiopathic Pulmonary Fibrosis1Department of Biomedical Engineering, The Ohio State University, Columbus, OH, United States, 2Department of Radiology, The Ohio State University Wexner Medical Center, Columbus, OH, United States, 3Division of Pulmonary, Critical Care, and Sleep Medicine, The Ohio State University Wexner Medical Center, Columbus, OH, United States
Synopsis
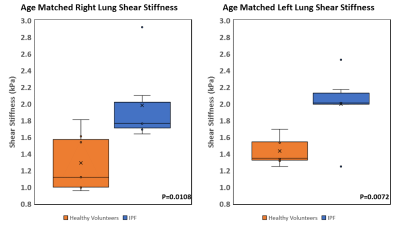
Interstitial lung diseases alter the mechanical properties of the lung parenchyma. Lung stiffness is a potential diagnostic marker. Standard breath hold techniques are difficult for patient with pulmonary disease. By using a free breathing Spin-Echo Dual-Density Spiral (SE-DDS) MRE sequence, a feasibility study was performed on 6 idiopathic pulmonary fibrosis (IPF) patients. It was found that the shear stiffness of IPF diseased lung is 2±0.48kPa for the right lung and 1.99±0.42kPa for the left lung which is significantly higher (P=0.0108 and P=0.0072) than healthy lungs, which has shear stiffness of 1.29±0.35kPa and 1.43±0.16kPa for right and left lungs, respectively.
Introduction
Lung diseases alter mechanical properties of the lung parenchyma1. The changes in mechanical properties such as stiffness of the lungs play an important role in patients’ ability to breathe normally. A class of diseases that typically alters the pulmonary parenchymal properties is known as interstitial lung disease (ILD). The most common interstitial lung disease is idiopathic pulmonary fibrosis. This debilitating illness affects 2.8-18 out of 100,000 people in Europe and North America with median survival of 2-4 years2. IPF is diagnosed by considering the clinical history, radiographic appearance of the lung parenchyma, and surgical pathology when available. Current guidelines suggest a multi-disciplinary approach to diagnosis including specialized ILD radiologist, thoracic pathologist, and pulmonary physicians. Usual interstitial pneumonia is the classic pathology found on lung biopsy specimens of IPF patients. This is characterized patchy areas of sub-pleural fibrosis intermixed with normal lung parenchyma that is termed spatial heterogeneity. In addition, there are areas of end stage scaring along with new areas of fibrosis, known as fibroblastic foci. Radiographic diagnosis of IPF is currently made possible by high resolution chest CT which contains ionizing radiation. Confirmatory open lung biopsy carries significant morbidity and potential mortality in this high risk population. Several studies have shown the feasibility of magnetic resonance elastography (MRE) to quantify the shear stiffness of the lungs1,3,4, which may enable the radiographic and clinical diagnosis of IPF. Patients suffering from IPF frequently suffers from breathlessness, which make breathhold techniques difficult. Therefore, the aim of this study was to investigate the change in stiffness measurements using free breathing MRE in healthy and patients with IPF.Methods
Six IPF patients (4 males and 2 females, 66.4±9.4 years old) were scanned using an approved IRB. Lung MRE scans covering both lungs were performed using a Spin-Echo Dual-Density Spiral (SE-DDS) sequence (Figure 1) in a 1.5T MR scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). Mechanical vibrations (Resoundant Inc, Rochester, MN, USA) of 50Hz were introduced into the lungs. The SE-DDS sequence was used due to its robustness to motion and short TE. With a TR of 1020ms and 10 spiral interleaves, the scan time was 1:30 minute for each motion-encoding direction (i.e. X, Y, Z). Depending on the size of the lung, 9 to 19 transverse slices were acquired with a thickness of 10mm. All scans were acquired during free breathing without the use of respiratory navigators and without the need of noble gas. The scan parameters included FOV of 45x45cm2, acquisition matrix of 128x128, and TE of 6.8ms. As shown in figure 1, two unipolar MEGs were placed around the 180° refocusing pulse with a period of 2.275ms (i.e. fractional encoding with a frequency of 220Hz combined) to achieve minimum possible TE. In addition, the MEGs were used as crushers for the 180° refocusing. To avoid motion/swirling artifacts that appear in spiral acquisition, a dual-density spiral was used in which the center of k-space was 4 times over sampled compared to the edges5. Additionally, non-Cartesian SPIRiT image reconstruction was used instead of non-uniform Fourier transform to further reduce spiral motion/swirling artifacts6. Lung density (LD) estimation scans were performed by using a GRE sequence involving four acquisitions with different TEs of 0.98, 1.62, 2.26, and 2.90ms to calculate T2* decay from which LD was estimated as described elsewhere4,7,8. LD measurement was used in the calculation of stiffness values. Since LD changes during the respiratory cycle, and to match the free-breathing MRE measurements, which is assumed to be an average signal across the respiratory cycle, LD scans were performed at the mid-point of the tidal volume in a given respiratory cycle under a 16-seconds breathhold. Lung shear stiffness was calculated by using the 2D direct inversion algorithm (MRElab software, Mayo Clinic, Rochester, Minnesota, USA)9.Results
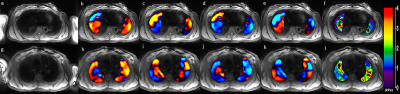
Figure 2 shows example of magnitude images, 4 snapshots of wave images, and a stiffness map of the right and left lungs of healthy volunteer and IPF patient. The wave images demonstrate excellent wave propagation during free breathing without any artifacts in the lungs. The stiffness map of the healthy volunteer (figure 2f) shows a mean shear stiffness of 1.35±0.88kPa (right lung) and 1.25±0.65kPa (left lung) across the entire slice. In addition, figure 2l shows the stiffness map of the IPF patient’s lung with a higher mean shear stiffness of 1.73±0.66kPa and 2.07±0.86kPa for the right and left lungs, respectively when compared to normal lungs. The mean shear stiffness values for the whole right and left lungs for all 6 patients was 2±0.48kPa and 1.99±0.42kPa, respectively. However, in another study conducted on healthy volunteers by Fakhouri et al. the mean shear stiffness values for the whole right and left lungs for all 7 age matched healthy volunteers was 1.29±0.35kPa and 1.43±0.16kPa, respectively. As shown in figure 3, there is a significant difference (P=0.0108 and P=0.0072 for right and left lung, respectively) in shear stiffness between healthy and IPF lungs.Discussion and Conclusion
This study has presented that the shear stiffness of IPF diseased lungs is significantly higher than healthy ones for both right and left lungs. Future studies of staging IPF based on stiffness will be performed and correlated to histopathology.Acknowledgements
We thank the Department of Biomedical Technology, King Saud University, Riyadh, Kingdom of Saudi Arabia, for providing scholarship to Faisal Fakhouri. Also we thank NIH-R01HL124096 and NCAI-18-11 for funding.References
1. Mariappan YK, Glaser KJ, Hubmayr RD, Manduca A, Ehman RL, McGee KP. MR elastography of human lung parenchyma: technical development, theoretical modeling and in vivo validation. J Magn Reson Imaging. 2011;33(6):1351-1361.
2. Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet (London, England). 2017;389(10082):1941-1952. doi:10.1016/S0140-6736(17)30866-8
3. Fakhouri F, Kolipaka A, … HD-P, 2018 U. Magnetic Resonance Elastography Derived Stiffness As a Novel Biomarker in the Cystic Fibrosis Lung. Pediatr Pulmonol. 2018;53:240-240. https://scholar.google.com/scholar?cluster=16990590023241398686&hl=en&oi=scholarr. Accessed February 28, 2019.
4. Mariappan YK, Glaser KJ, Levin DL, et al. Estimation of the absolute shear stiffness of human lung parenchyma using 1H spin echo, echo planar MR elastography. J Magn Reson Imaging. 2014;40(5):1230-1237.
5. Meyer CH, Zhao L, Lustig M, et al. Dual-Density and Parallel Spiral ASL for Motion Artifact Reduction. Proc ISMRM. 2011;64(3):3986. doi:DOI: 10.13140/RG.2.2.17527.62880
6. Lustig M, Pauly JM. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn Reson Med. 2010;64(2):457-471. doi:10.1002/mrm.22428
7. Holverda S, Theilmann RJ, Sá RC, et al. Measuring lung water: Ex vivo validation of multi‐image gradient echo MRI. J Magn Reson Imaging. 2011;34(1):220-224.
8. Theilmann RJ, Arai TJ, Samiee A, et al. Quantitative MRI measurement of lung density must account for the change in T 2* with lung inflation. J Magn Reson Imaging. 2009;30(3):527-534.
9. Manduca A, Oliphant TE, Dresner MA, et al. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Med Image Anal. 2001;5(4):237-254. doi:10.1016/S1361-8415(00)00039-6
Figures