3325
Development of in vivo human brain DTI-MRE1Richard and Loan Hill Department of Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 2Department of Bioengineering, University of Illinois at Urbana-Champaign, Urbana-Champaign, IL, United States
Synopsis
Simultaneous acquisition of diffusion tensor imaging (DTI) and magnetic resonance elastography (MRE) has been proven feasible in previous pre-clinical studies. DTI-MRE requires the identification of experimental parameter ranges that depend on the specific target. Here we identify valid experimental parameter sets in simulations for in vivo human brain applications and the DTI-MRE technique is implemented by modifying a single-shot spin-echo EPI sequence. A good agreement was found between DTI-MRE and conventional acquisitions. DTI-MRE may increase the clinical acceptance of MRE and DTI by providing co-registered diffusion and tissue mechanical property maps within the scan time of a conventional DTI acquisition.
Introduction
Magnetic Resonance Elastography (MRE) and Diffusion Tensor Imaging (DTI) are noninvasive motion-encoding MRI techniques that are capable of determining tissue stiffness and diffusion properties, respectively. Tissue stiffness and the diffusive behavior of water in tissue represent complementary diagnostic information for a variety of pathologic conditions, such as, cancer, fibrosis and neurodegeneration.1,2 In MRE tissue vibrations are encoded in the phase of the complex-valued MRI signal, while DTI uses the magnitude for measuring diffusive information. Both techniques have in common balanced magnetic field gradients that are applied in 3D space for motion encoding. We have previously shown using a mouse brain model that DTI and MRE information can be acquired simultaneously by our developed approach named DTI-MRE.3,4 Compared to sequential DTI and MRE acquisitions, DTI-MRE reduces scan time by a factor of ~2 and provides images that are immediately co-registered. However, DTI-MRE requires the identification of experimental parameters with the best compromise between maximizing motion encoding efficiency and minimizing intravoxel phase dispersion as the latter may cause interference in the vibration and diffusion measurements. In simulations we identified optimal experimental parameters of in vivo human brain DTI-MRE and present these together with preliminary results demonstrating the feasibility.Methods
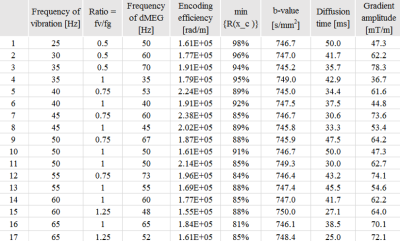
The conditions in simulations used a b-value within range of [745,750] , an encoding efficiency of vibration above 1.5x105 rad/m, vibration frequencies within range of [20,70] Hz, and a separation time between motion encoding gradients (MEG) selected to be integer multiples of vibration periods. Finally, an equation described previously,3 is used to assess the extent of signal loss in magnitude images due to intravoxel phase dispersion (IVPD), which may interfere with DTI analysis. We further require for the experimental parameters that the IVPD-induced signal loss must be less than 20 %. The DTI-MRE validation study on in vivo human brain was approved by our Institutional Review Board (Protocol # 2018-1042). Our initial study was a comparison between in vivo human brain DTI-MRE and conventional acquisitions at 3T MRI using a human scanner (Prisma, Siemens). A single-shot, spin-echo echo planar imaging (SE-EPI)-based MRE sequence was modified with the gradient waveforms and diffusion time set to fulfill the timing condition for simultaneous acquisitions in DTI-MRE. We used single trapezoid gradient waveforms in the sequence. We implemented DTI-MRE using 24 directions that are equally distributed in 3D space and grouped into eight sets of three directions with minimized linear dependency.3 Each MRE phase offset is linked to one of eight gradient sets. The vibration frequency, vibration encoding efficiency, b-value, in-plane resolution, slice thickness and TR/TE are 50 Hz, 2.2x105 rad/m, 780.7 s/mm2, 2 mm, 4 mm and 6000/68 ms, respectively. Conventional spin-echo 64-direction DTI and MRE were compared with DTI-MRE. Diffusion property maps and the complex shear modulus G* were calculated as previously described.3 The Spearman correlation r was determined voxel-wise between DTI-MRE and conventional methods in a central brain slice excluding fluid-filled ventricles.Results
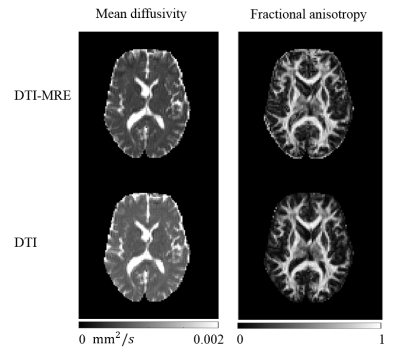
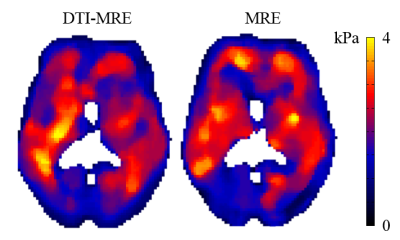
Figure 1 shows 17 sets of experimental parameters that meet the simulation conditions. Figures 2 and 3 illustrate a central brain slice of diffusion and mechanical properties, respectively. In this slice the voxel-wise Spearman correlation coefficients of fractional anisotropy (FA), mean diffusivity (MD) and |G*| are 0.99, 0.99 and 0.65, respectively.Discussion
Our feasibility study shows a good correlation between in vivo human brain DTI-MRE and conventional methods. The simulation results suggest the potential use of fractional encoding in future experiments. We will further optimize the experimental parameters of in vivo human brain DTI-MRE and conduct a pilot study using a larger sample size in the next months. DTI-MRE has the potential to increase the clinical acceptance of MRE and DTI by providing fast acquisitions and diffusion and tissue mechanical property maps that are immediately co-registered.Summary
A good correlation was found between rapid in vivo human brain DTI-MRE and conventional acquisitions based on diffusion and tissue mechanical property maps.Acknowledgements
This work is supported by NIH R21EB026238 Adding MRE to DTI for free.References
1. Hirsch S, Braun J, Sack I. Magnetic Resonance Elastography: Physical Background and Medical Application. Wiley-VCH 2017.
2. Susumu M. Introduction to Diffusion Tensor Imaging. Elsevier 2007.
3. Yin Z, Kearney SP, Magin RL, Klatt D. Concurrent 3D Acquisition of Diffusion Tensor Imaging and Magnetic Resonance Elastography Displacement Data (DTI-MRE): Theory and In Vivo Application. Magnetic Resonance in Medicine 2017; 77(1): 273-284.
4.Yin Z, Magin RL, Klatt D. Simultaneous MR elastography and diffusion acquisitions: Diffusion-MRE (dMRE). Magnetic Resonance in Medicine 2014; 71(5): 1682-1688.
Figures