3309
Simultaneous Fat-Water MR Elastography Imaging using Distortion-free DIADEM-EPI1Radiology, Mayo Clinic, Rochester, MN, United States, 2Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN, United States
Synopsis
Simultaneous fat-water EPI-MR elastography imaging was developed for acquisition of the brain (water) and skull (bone marrow fat) MRE motion. Compared to our previous dual-saturation and dual-sensitivity motion encoding (DSDM) technique, the proposed method minimize the EPI distortion and misalignment between skull and brain. This technique could offer a unique opportunity to quantify the skull-brain mechanical decoupling performance modulated by the pia-arachnoid complex, and its potential alterations in response to repetitive head impacts (RHI) in our future studies.
Introduction
Repetitive head impacts (RHI) sustained in contact sports is a growing public health issue. Growing evidence indicates that if sustained repetitively, subconcussive low levels of head impact can cause significant head injury and increase the concussion susceptibility.1 To elucidate injury mechanisms of RHI, many efforts have studied how head impacts affect the skull-brain (SB) biomechanics.2-4 Recently, we have developed an MR elastography (MRE)-based dual-saturation and dual-sensitivity motion encoding (DSDM) technique that directly measures in vivo skull-brain motion,5 and have shown that the assessment of SB mechanical decoupling performance can be used to detect RHI-induced injury to the protective pia-arachnoid complex in humans.6 In previous studies, the MRE data from the brain (water) and skull (bone marrow fat) signal were acquired in serial but separate scans. The patient head movement between the two separate scans would affect the alignment of the skull and brain images and the accuracy of quantifying the relative skull and brain motion. Recently, a couple of point-spread-function mapping7 based distortion‐free EPI imaging techniques, such as DIADEM 8,9 and tilted-CAIPI 10,11, have been developed to remove the distortion and/or the chemical shift of the fat for diffusion imaging. Here, we extended these techniques to the multi-band EPI-MRE sequence12, termed multi-band DIADEM-MRE (or MB-DIADEM-MRE) to allow for simultaneous acquisition of the brain and skull MRE motion. The purpose of this preliminary study was to demonstrate the feasibility of the proposed MB-DIADEM-MRE technique.Methods
Pulse sequence: The MB-DIADEM-MRE sequence is based on a multi-band spin-echo EPI-MRE sequence.12 The additional spin-warp phase-encoding gradient was added right before the EPI acquisition.7,13 The fat saturation module, including fat saturation RF pulse and slice-selection gradient reversal,14 was disabled to preserve the fat signal from the skull bone marrow. The dual-sensitivity motion encoding was added on both sequences in order to reliably unwrap the skull and brain phase images.5Volunteer experiments: With the IRB approval and written informed consent, three healthy volunteers were scanned on a high-performance compact 3T scanner15,16 using a Nova Medical 32-channel receiver coil. The MB-DIADEM-MRE data was acquired at 3-mm isotropic resolution with TR/TE=1584/50.3 ms; FOV=24 cm; 80×80 matrix; 38 contiguous 3-mm-thick axial slices; 3x in-plane acceleration; 2x multiband acceleration, 268 μs echo spacing, 60-Hz mechanical vibrations, 6 motion encoding directions, 4 phase offsets; 8 shots for each DIADEM dataset and 5:07 minutes scan time. A separate tilted-CAIPI calibration data was acquired in 12 seconds with the identical protocol except for TR/TE=1000/15.5 ms, no multiband acceleration, no MRE encoding gradient, and 12 shots. For comparison, a standard DSDM multi-slice EPI-MRE was scanned with the matched FOV, resolution, and MRE parameters. Of note in DSDM-MRE, the water and fat images were acquired in the same series, but separately in two sequential acquisitions.
Image reconstruction and post-processing: For the MB-DIADEM-MRE, after the vendor-provided multiband EPI reconstruction of each shot, the tilted-CAIPI reconstruction was followed to unfold the DIADEM dataset up to 2 times. This was able to cover an off-resonance frequency range of up to 1120 Hz and a distortion-free water-and-fat signal can be obtained from the DIADEM data. For the DSDM-MRE, the water and fat MRE data were combined in the complex image domain using phase preserving complex combination as described in the previous study.5 The MRE phase from both methods was unwrapped using the dual sensitivity method.5 The skull mask is automatically generated from T1W anatomic images acquired at 1x1x1.2 mm3 resolution. The T1W images were first registered to the MRE space using SPM, and then the same transformation matrix was applied to the skull mask.
Results
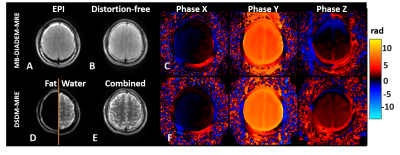
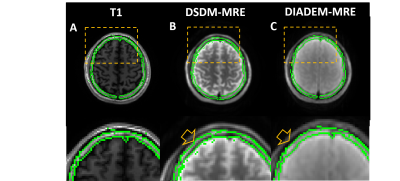
Figure 1 compares the magnitude and unwrapped phase images between MB-DIADEM-MRE and DSDM-MRE in one representative volunteer. The top row shows the EPI-like images and distortion-free images generated from the same DIADEM dataset.17 The large fat chemical shift can be seen on the EPI-like image (Fig. 1A) that was correctly reconstructed on the distortion-free image (Fig. 1B). The bottom row shows the skull and brain images (Fig. 1D) acquired with DSDM-MRE, and their combined image (Fig. 1E). The MRE phase images from both methods (Fig. 1C, F) were successfully unwrapped using dual-sensitivity method even on the y-direction motion with a large peak-to-peak phase-amplitude over 6π.Figure 2 shows a representative slice of the skull ROI (green contours) automatically generated from T1 anatomic images (Fig. 2A). The T1 skull ROI was overlaid on the combined magnitude image acquired from DSDM-MRE (Fig. 2B) and on the magnitude of DIADEM-MRE (Fig. 2C). Given the skull layer is only 2-3 pixels wide, even a subtle distortion or misalignment due to the patient movement could cause the mismatch of the mask (arrow on Fig. 2B) on the DSDM-MRE images.
Discussion and Conclusion
Both the water and fat MRE can be simultaneously acquired with the proposed multiband DIADEM-MRE. The registration accuracy of the skull mask was improved. This technique could offer a unique opportunity to quantify the skull-brain mechanical decoupling performance modulated by the pia-arachnoid complex, and its potential alterations in response to RHI in our future studies. Furthermore, the water/fat DIADEM-MRE could be used to improve the performance of the breast EPI-MRE by acquiring wave information from both fibroglandular tissue and fatty tissue.Acknowledgements
This work was supported by grants from the National Institute of Health (R01 EB001981, R01 EB010065, R01 NS113760, and NIH U01 EB024450)References
1. Mainwaring L, Ferdinand Pennock KM, Mylabathula S, Alavie BZ. Subconcussive head impacts in sport: A systematic review of the evidence. Int J Psychophysiol 2018;132(Pt A):39-54.
2. Zou H, Schmiedeler JP, Hardy WN. Separating brain motion into rigid body displacement and deformation under low-severity impacts. Journal of Biomechanics 2007;40(6):1183-1191.
3. Feng Y, Abney TM, Okamoto RJ, Pless RB, Genin GM, Bayly PV. Relative brain displacement and deformation during constrained mild frontal head impact. J R Soc Interface 2010;7(53):1677-1688. 4. Scott GG, Coats B. Microstructural Characterization of the Pia-Arachnoid Complex Using Optical Coherence Tomography. Ieee T Med Imaging 2015;34(7):1452-1459.
5. Yin Z, Sui Y, Trzasko JD, Rossman PJ, Manduca A, Ehman RL, Huston J, 3rd. In vivo characterization of 3D skull and brain motion during dynamic head vibration using magnetic resonance elastography. Magn Reson Med 2018.
6. Yin Z, Sui Y, Manduca A, Romano AJ, Ehman RL, III JH. MR Elastography (MRE)-assessed skull-brain coupling is affected by sports-related repetitive head impacts (RHI). ISMRM Proceeding 2019;27:3005.
7. Robson MD, Gore JC, Constable RT. Measurement of the point spread function in MRI using constant time imaging. Magnetic resonance in medicine 1997;38(5):733-740.
8. In MH, Speck O. Highly accelerated PSF-mapping for EPI distortion correction with improved fidelity. MAGMA 2012;25(3):183-192.
9. In MH, Tan ET, Trzasko JD, Shu Y, Kang D, Yarach U, Tao S, Gray EM, Huston J, 3rd, Bernstein MA. Distortion-free imaging: A double encoding method (DIADEM) combined with multiband imaging for rapid distortion-free high-resolution diffusion imaging on a compact 3T with high-performance gradients. J Magn Reson Imaging 2019.
10. Dong Z, Wang F, Reese TG, Manhard MK, Bilgic B, Wald LL, Guo H, Setsompop K. Tilted-CAIPI for highly accelerated distortion-free EPI with point spread function (PSF) encoding. Magn Reson Med 2019;81(1):377-392.
11. Hu Z, Wang Y, Dong Z, Guo H. Water/fat separation for distortion-free EPI with point spread function encoding. Magn Reson Med 2019;82(1):251-262.
12. Sui Y, Yin Z, Rossman PJ, Murphy MC, Trzasko JD, Scott JM, Glaser KJ, McGee KP, Bernstein MA, Ehman RL, III JH. Fast Brain MR Elastography Using a Simultaneous Multislice EPI Acquisition on a Compact 3T Scanner. ISMRM Proceeding 2019:3971.
13. Zeng H, Constable RT. Image distortion correction in EPI: comparison of field mapping with point spread function mapping. Magn Reson Med 2002;48(1):137-146.
14. Park HW, Kim DJ, Cho ZH. Gradient Reversal Technique and Its Applications to Chemical-Shift-Related Nmr Imaging. Magnetic Resonance in Medicine 1987;4(6):526-536.
15. Weavers PT, Shu YH, Tao SZ, Huston J, Lee SK, Graziani D, Mathieu JB, Trzasko JD, Foo TKF, Bernstein MA. Technical Note: Compact three-tesla magnetic resonance imager with high-performance gradients passes ACR image quality and acoustic noise tests. Medical Physics 2016;43(3):1259-1264.
16. Foo TKF, Laskaris E, Vermilyea M, Xu M, Thompson P, Conte G, Van Epps C, Immer C, Lee SK, Tan ET, Graziani D, Mathieu JB, Hardy CJ, Schenck JF, Fiveland E, Stautner W, Ricci J, Piel J, Park K, Hua Y, Bai Y, Kagan A, Stanley D, Weavers PT, Gray E, Shu Y, Frick MA, Campeau NG, Trzasko J, Huston J, 3rd, Bernstein MA. Lightweight, compact, and high-performance 3T MR system for imaging the brain and extremities. Magn Reson Med 2018;80(5):2232-2245.
17. In MH, Posnansky O, Speck O. High-resolution distortion-free diffusion imaging using hybrid spin-warp and echo-planar PSF-encoding approach. Neuroimage 2017;148:20-30.
Figures