3293
Super-resolution reconstruction of single-PLD pseudo-continuous ASL images1imec - Vision Lab, Department of Physics, University of Antwerp, Antwerp, Belgium, 2C.J. Gorter Center for High Field MRI, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 3Leiden Institute of Brain and Cognition, Leiden University, Leiden, Netherlands
Synopsis
Super-resolution reconstruction (SRR) allows for high-resolution image reconstruction from a set of low-resolution multi-slice images with different orientations. Arterial spin labeling (ASL) is an interesting albeit complicated candidate for SRR, as it relies on subtraction. SRR-ASL can be performed on low-SNR subtracted or on low-contrast unsubtracted ASL data. Different ASL-SRR implementations were applied to single-PLD PCASL data and validated against traditional ASL-scans. Combining motion correction, super-resolution post-processing and pairwise subtraction of label-control pairs in a single framework yielded comparable CBF maps as with traditional HR-ASL. Furthermore, in certain slices, SRR-ASL appears to reconstruct the underlying anatomical structure with higher fidelity.
Introduction
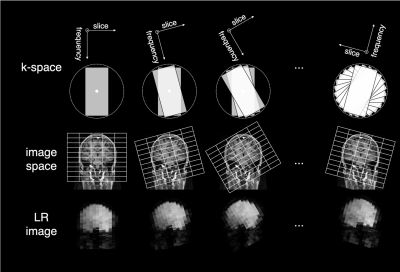
Super-resolution reconstruction (SRR) aims at improving the trade-off between spatial resolution, SNR and scan time by using iterative reconstruction techniques on a set of 2D-multi-slice images with low through-plane resolution, where each of those images samples the underlying high-resolution (HR) object in a unique way, ultimately allowing an isotropic HR 3D-reconstruction1,2,3. An efficient sampling method is to rotate the slice-encoding direction around the phase-encoding direction over a wide range of angles (Figure 1). From such a set of low-resolution (LR) multi-slice MR-images, it has been shown that high-frequency information can be recovered with SRR post-processing, ultimately resulting in HR images or parameter maps4,5,6.Arterial spin labeling (ASL) is an interesting albeit complicated candidate for SRR. The requirement in SRR of acquiring multiple images fits naturally with ASL, as there is an inherent need for averaging7. Furthermore, compared to acquiring HR multi-slice data, acquisition of LR multi-slice images (i.e., fewer, thicker slices) is beneficial in terms of keeping the effectiveness and level of background suppression more constant over the volume and to limit the variation of the effective post-labeling delay. This could ultimately result in a significantly increased SNR, similarly as found for multiband ASL8.
A major difference of an ASL-SRR implementation compared to other MRI modalities is the subtraction step in ASL. For symmetry reasons, for each rotation both a label and a control image have to be acquired. Moreover, it has to be decided to perform SRR either on the subtracted or on the unsubtracted data. In this study, different ASL-SRR implementations were applied to single-PLD pseudo-continuous ASL (PCASL) data and validated against traditional ASL-scans.
Methods
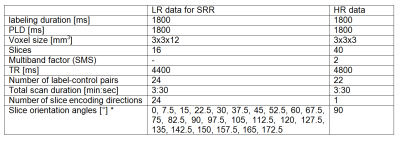
Data acquisitionSingle-shot EPI multi-slice single-PLD PCASL data was acquired from a healthy volunteer (informed consent was obtained and study was approved according to local ethics guidelines). Both a set of LR-PCASL data, suitable for SRR, and a conventional, HR-PCASL dataset was acquired, serving as a reference for comparison. Acquisition settings are shown in Table 1. Furthermore, a HR proton density image was acquired for absolute quantification.
Post-processing pipelines
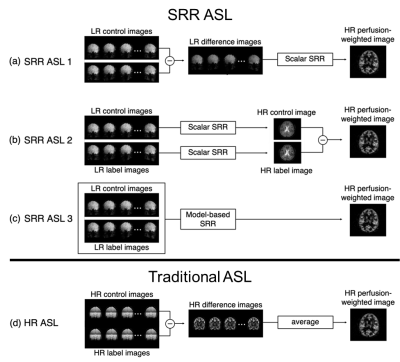
Three versions of combining the SRR step and the subtraction ASL step were implemented (Figure 2a-c). SRR postprocessing was performed as regularized nonlinear least squares estimation:
$$(\hat{\boldsymbol{x}},\hat{\boldsymbol{\theta}})=\arg\min_{\boldsymbol{x},\boldsymbol{\theta}}\{||\boldsymbol{A}_\boldsymbol{\theta}f(\boldsymbol{x})-\boldsymbol{y}||_2^2+\lambda||\boldsymbol{\Delta}\boldsymbol{x}||_2^2\},$$ with $$$\boldsymbol{x}$$$ and $$$\boldsymbol{\theta}$$$ vectors representing the HR image and unintended motion parameters to be estimated9, respectively, $$$\boldsymbol{y}$$$ a vector representing the input LR data, $$$\boldsymbol{A_\theta}$$$ the SRR sampling matrix relating the HR image to each of the differently acquired LR images and $$$f(\cdot)$$$ a model relating the HR perfusion-weighted image to label-control image pairs (SRR-ASL-3), or the identity operator (SRR-ASL-1/2). The second term depicts Laplacian regularization, common for SRR4,5, controlled by a weighting factor $$$\lambda$$$. Regularization weighting was optimized for each pipeline while ensuring a fixed ratio between the data fidelity term and regularization term. In case of traditional ASL, conventional steps of image registration, pair-wise subtraction and subsequent averaging were followed (Figure 2d). Finally, HR CBF maps were quantified using the recommended PCASL quantification model6.
Results and Discussion
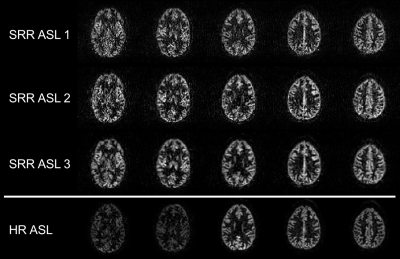
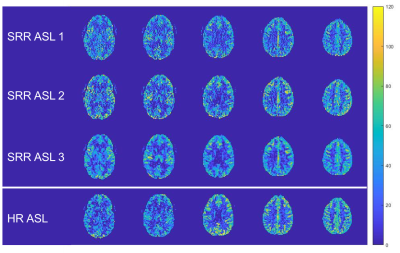
The reconstructed HR (3x3x3mm3) perfusion-weighted images using the three SRR ASL methods and the traditional ASL pipeline are shown in Figure 3 and the quantified HR CBF maps in Figure 4.When comparing the different SRR-ASL approaches, the model-based SRR-ASL-3 pipeline qualitatively outperforms the other two SRR pipelines (Fig. 3 and 4). In the case of SRR-ASL-1, SRR is performed on low-SNR difference images, potentially explaining the higher noise-level in the reconstructed HR perfusion-weighted images. For SRR-ASL-2, the subtraction step is not taken into account in the two separate scalar SRRs, making it more vulnerable to registration errors. Moreover, regularization is performed on the unsubtracted images, which show less high-contrast details than perfusion-weighted images. Motion correction, super-resolution post-processing and pairwise subtraction of label-control pairs are performed in one single optimization framework in SRR-ASL-3, making it less susceptible to error propagation while maintaining robustness because regularization is also performed on the final HR perfusion-weighted outcome image.
When comparing SRR-ASL-3 to the traditional HR-ASL experiment, the benefit of acquiring LR data for SRR-ASL shows in terms of SNR. The HR SRR-ASL-3 images show comparable signal intensities in all slices, while for traditional HR-ASL later acquired slices suffer from low SNR due to long effective PLDs and limited background suppression. For such slices (first two in Figure 3), SRR-ASL-3 appears to reconstruct the underlying anatomical structure with higher fidelity compared to traditional HR-ASL.
In terms of absolute quantification, the CBF estimates resulting from the SRR-ASL pipelines are in the same range as those obtained from the traditional ASL experiment. However, there are some clear regional differences (Figure 4). This could potentially be caused by accumulation of information from different effective PLDs in each voxel stemming from the different slice orientations of the LR images, whereas the traditional HR-ASL has a different PLD for each slice. In future work, we will try to exploit the presence of multiple effective PLDs by incorporating the quantification step within the model-based SRR framework.
Conclusion
The feasibility of super-resolution reconstruction of 2D-multi-slice single-PLD PCASL data was demonstrated in this work. The technique shows clear promise for whole-brain high-resolution multi-slice PCASL imaging, although comparison against 3D readout strategies is needed.Acknowledgements
PB is predoctoral fellow of the Research Foundation Flanders (FWO), Grant 1S69918N. JS and AJD gratefully acknowledge support of the European Space Agency (ESA) and BELSPO Prodex through the BrainDTI project. MJPvO acknowledges support from the EU (Horizon2020, CDS-QUAMRI, Project number 634541), by the Dutch Heart Foundation and the Netherlands Organisation for Scientific Research (NWO), as part of their joint strategic research programme: "Earlier recognition of cardiovascular diseases (project: Brain@Risk) and the Netherlands Organisation for Scientific Research (NWO) (VICI-project; nr. 016.160.351).References
- Plenge E, Poot DHJ, Bernsen M, Kotek G, Houston G, Wielopolski P, van der Weerd L, Niessen WJ, Meijering E. Super-resolution methods in MRI: Can they improve the trade-off between resolution, signal-to-noise-ratio, and acquisition time? Magn Reson Med 2012;68:1983–1993.
- Shilling RZ, Robbie TQ, Bailloeul T, Mewes K, Mersereau RM, Brummer ME. A super-resolution framework for 3-D imaging using 2-D multislice MRI. IEEE Trans Med Imaging 2009;28:633–644.
- Poot DHJ, Van Meir V, Sijbers J. General and efficient super-resolution method for multi-slice MRI. Med Image Comput Comput Assist Interv 2010(Pt 1); 615–622.
- Poot DHJ, Jeurissen B, Bastiaensen Y, Veraart J, Van Hecke W, Parizel PM, Sijbers J. Super-resolution for multislice diffusion tensor imaging. Magn Reson Med 2013;69:103–113.
- Van Steenkiste G, Poot DHJ, Jeurissen B, den Dekker AJ, Vanhevel F, Parizel PM, Sijbers J. Super‐resolution T1 estimation: Quantitative high resolution T1 mapping from a set of low resolution T1‐weighted images with different slice orientations. Magn Reson Med 2017;77(5):1818-1830.
- Van Steenkiste G, Jeurissen B, Veraart J, den Dekker AJ, Parizel PM, Poot DHJ, Sijbers J. Super‐resolution reconstruction of diffusion parameters from diffusion‐weighted images with different slice orientations. Magn Reson Med 2016;75(1):181-195.
- Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJP, Wang DJJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015;73:102-116.
- Suzuki Y, Okell TW, Chappell MA, van Osch MJP. A framework for motion correction of background suppressed arterial spin labeling perfusion images acquired with simultaneous multi‐slice EPI. Magn Reson Med 2019;81(3):1553-1565.
- Beirinckx Q, Jeurissen B, Verhoye M, den Dekker AJ, Sijbers J. Super-resolution T1 mapping with integrated motion compensation in a joint maximum likelihood framework. 36th Annual Scientific Meeting of the European Society for Magnetic Resonance in Medicine & Biology (ESMRMB), Rotterdam, The Netherlands, vol. 32 (Suppl. 1), no. S14.05: Magn Reson Mater Phy, 2019.
Figures