3288
Volumetric, multi B-value, multi-delay ASL using 3D single-shot spirals, expanded encoding models, and low rank reconstruction1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
In this work, we investigate a strategy to acquire single-shot, multi b-value, multi-delay ASL scans using the combination of an undersampled spiral in-out 3D fast spin echo readout with reconstructions enforcing a low rank constraint with an expanded encoding model. Images are demonstrated in healthy volunteers, acquiring single 3D ASL images in as short as 11s.
Purpose
Permeability provides insight to blood brain barrier (BBB) dysfunction, which is associated with aging and diseases. However measuring BBB permeability is challenging even when using intravascular exogenous contrast agents, such as Ferumoxytol1. Recent work has proposed a combination of principles from intravoxel incoherent motion (IVIM) with multi-delay arterial spin labeling (MD-ASL) to extract BBB water permeability without the need for exogenous contrast (BBB-ASL)2. This revolves around (1) being able to separate intra and extra vascular signals using diffusion and flow differences, and (2) observing the passage of tagged intravascular water to the extravascular compartment. With the combination of IVIM and MD-ASL, the number of images required to obtain accurate fitting is generally high due to the product of the number of b-values and delays required.Previous studies have demonstrated promising results with diffusion prepared MD-ASL using single-shot 2D echo-planar imaging (EPI) with limited spatial coverage and resolution to overcome scan time limitations. A recent study also proposed to measure BBB permeability with 3D gradient and spin echo readout and regularized fitting3. However, with the need for multiple phases, the total acquisition time is relatively long and the model assumptions may not hold true in cases of disease. Spiral trajectories offer higher sampling efficiency and beneficial flow and motion properties compared to EPI based trajectories. This study aimed to perform diffusion prepared ASL with 3D single-shot, fast spin echo (FSE) and stack-of-spirals readout harnessing a combination of accelerated acquisition and reconstruction strategies.
Methods
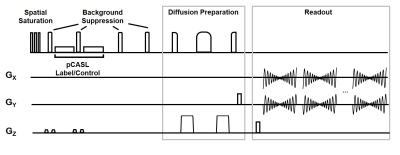
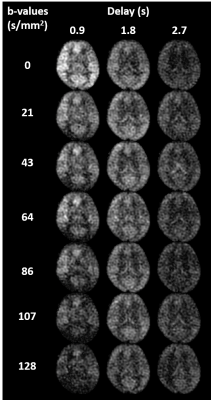
Single-shot spiral diffusion preparation MD-ASL: Figure 1 shows an overview of the proposed imaging sequence with pseudo-continuous arterial spin labeling (pCASL), interleaved background suppression4, and 3D single-shot fast spin echo (FSE) stack of spirals readout. Images are sampled at unique b-values and delays for multiple repetitions. To reduce blurring, allow for acceleration, and center the spin echo5, a variable density single-shot spiral in-out trajectory is used. Fat saturation and adiabatic diffusion preparations are applied immediately before the imaging readout.Image acquisition in healthy volunteers: Seven healthy volunteers were imaged in this IRB approval study at 3T (GE Signa Premier, GE Healthcare, Waukesha, WI) with a 48-channel head coil. Diffusion-prepared MD-ASL was acquired with three post-labeling delays (0.9, 1.8, 2.7 sec) and seven b-value encodings (0, 21, 43, 64, 86, 107, 128 s/mm2) using a 3D single-shot stack-of-spiral readout (in-plane matrix = 64 × 64; resolution=4x4x4mm, field-of-view = 260x260x192 mm; bandwidth = 125 kHz; TR = 5567 ms; TE = 37.2 ms; 40 slices; slice thickness = 4 mm; total scan time = 161s for 28 unique images). Additional multi-echo 3D gradient echo images were collected with delayed and ultrashort echo times (TE=0.1,1.2,2.4,4.8ms) to enable field and coil sensitivity mapping.
Reconstruction: Images were reconstructed offline using GPU accelerated reconstructions (SigPy, Open Source). Sensitivity maps were derived from low resolution UTE images, allowing for near complete removal of B1- phase. Field maps were generated from the phase difference between the gradient echo UTE and delayed echo time images. Image reconstruction was performed over all delay times and b-values simultaneously in an iterative model based reconstruction incorporating a local low rank constraint across all images and an expanded encoding model incorporating the field maps. Time segmentation6 was utilized to reduce the computational burden of the encoding model.
Results
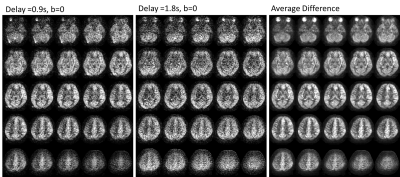
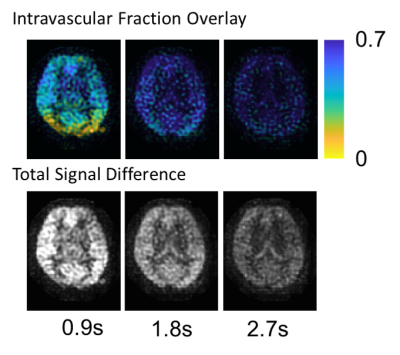
Images were successfully acquired in all volunteers with consistent image quality, as expected in a healthy subject pool. Figure 2 shows example images for a single slice, while Figure 3 shows the 3D volumetric nature of the data. Despite each image requiring only 11s of acquisition, high image quality is observed. Initial results using the data for intravascular volume fraction mapping is shown in Figure 4. Interestingly, only mono-exponential signal decay is observed (as fit), suggesting pulse sequence dependencies, such as M1, on the IVIM modeling.Discussion and Conclusions
Our results suggest that 3D single-shot ASL is feasible, producing images of comparable quality to that of multi-shot, multiple average scan. Single-shot 3D encoding enables new acquisition strategies that are not currently possible. This includes the potential for the proposed multi-b value, multi-delay sequence but also dynamic acquisitions for imaging functional neuroactivation and response physiologic challenges. While not proven, the multi-shot acquisition should also be more robust to motion allowing for rejection of corrupt volumes and motion correction. Work is ongoing to evaluate this technique in diseased patient pool and to develop strategies for optimal quantification for BBB permeability.Acknowledgements
The authors wish to acknowledge support from GE Healthcare, and a Research and Development Grant from the Departments of Radiology and Medical Physics, University of Wisconsin.References
1. Rivera-Rivera LA, Schubert T, Knobloch G, Turski PA, Wieben O, Reeder SB, Johnson KM. Comparison of ferumoxytol-based cerebral blood volume estimates using quantitative R 1 and R2* relaxometry: Ferumoxytol-Based Cerebral Blood Volume Estimates. Magn Reson Med. 2018 Jun;79(6):3072–3081.
2. Shao X, Ma SJ, Casey M, D’Orazio L, Ringman JM, Wang DJJ. Mapping water exchange across the blood-brain barrier using 3D diffusion-prepared arterial spin labeled perfusion MRI. Magn Reson Med. 2018 Dec 18;
3. Wengler K, Bangiyev L, Canli T, Duong TQ, Schweitzer ME, He X. 3D MRI of whole-brain water permeability with intrinsic diffusivity encoding of arterial labeled spin (IDEALS). NeuroImage. 2019 Apr 1;189:401–414.
4. Maleki N, Dai W, Alsop DC. Optimization of background suppression for arterial spin labeling perfusion imaging. Magma N Y N. 2012 Apr;25(2):127–133. PMCID: PMC3671066
5. Li Z, Schär M, Wang D, Zwart NR, Madhuranthakam AJ, Karis JP, Pipe JG. Arterial spin labeled perfusion imaging using three-dimensional turbo spin echo with a distributed spiral-in/out trajectory. Magn Reson Med. 2016 Jan;75(1):266–273. PMID: 25754947
6. Sutton BP, Noll DC, Fessler JA. Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE Trans Med Imaging. 2003 Feb;22(2):178–188. PMID: 12715994
Figures