3286
Investigating the Repeatability of Cerebrovascular Reactivity Using Single and Multi-PLD PCASL1Department of Radiology, Stanford University, Stanford, CA, United States, 2Department of Radiology, Shuang-Ho Hospital, Taipei Medical University, Taipei, Taiwan, 3GE Healthcare, Menlo Park, CA, United States
Synopsis
This work investigates the repeatability of CVR (induced by acetazolamide) using ASL on a healthy cohort. Single and multi-PLD PCASL data were collected before and after the administration of acetazolamide in two identical sessions. Results showed that the mean CVR of both ASL techniques were between 20% and 60% and not significantly different. Measurement using single-PLD PCASL demonstrated higher repeatability based on a lower coefficient of variation and a higher intraclass correlations.
Introduction
Cerebrovascular reactivity (CVR) reflects the change in cerebral blood flow (CBF) in response to a vasoactive stimulus. Studies have demonstrated that CVR has the potential to become an important biomarker to assess the health of brain tissues and cerebral vascular diseases1. CVR can be measured in a stress-test where a stimulus, such as acetazolamide (ACZ), is administered to alter the blood flow from the baseline condition. Whilst single-PLD PCASL has been recommended as the standard implementation for CBF quantification2, multi-PLD techniques offer the advantage of controlling ATT variations, which is observed in CVR experiments3,4. Despite the application of ASL in quantifying CVR, it is not clear whether the single or multi-PLD technique is more suitable and its repeatability has not been examined.Here, we investigate the repeatability of CVR (induced by ACZ) of single and multi-PCASL on a healthy cohort data from two identical MRI sessions. Mean CBF and CVR of the whole brain were compared. Inter-session and inter-subject variations of the repeated measurements were assessed using coefficient of variation (CV) and interclass correlation coefficient (ICC).
Methods
All procedures were approved by the local institutional review board of Stanford University. MRI data were collected from eight healthy volunteers (mean age 39 ± 14 years, five female) after they had given written informed consent. Each subject underwent ACZ administration at a dose of 15 mg/kg of body weight with a maximum of 1g during the MRI procedures. All MRI experiments were conducted twice separated by an average of 9 days on a 3T PET/MRI scanner (SIGNA; GE Healthcare, Waukesha, WI, USA). In each experiment, single and multi-PLD ASL scans were performed before and 15 minutes after ACZ administration. The single-PLD PCASL was conducted using GE’s ASL product sequence parameters with PLD=2025ms, bolus duration=1450ms, interleave arms=6, and NEX=35. The multi-PLD PCASL was implemented using the same parameters as in the single-PLD except for PLD=700, 1325, 1950, 2575, 3200ms, bolus duration=2000ms, interleave arms=4, and NEX=1. A proton density image was acquired using a saturation recovery technique with TR=2000ms and other parameters identical to the PCASL sequence, and a 3D T1-weighted structural image was acquired.Absolute CBF and ATT from both ASL techniques was estimated by fitting the general kinetic model to the ASL difference data using the FSL tool BASIL6, assuming the inversion efficiency of 85%, signal loss due to background suppression of 25%, and brain/blood partition coefficient of 0.9 ml/g. All CBF images were transformed to the standard MNI space using the FSL tool FLIRT and FNIRT7. CVR is defined as the percentage of change in CBF from baseline CBF.
Two-tailed paired t-tests were conducted to compare the whole-brain CBF and CVR between sessions and ASL techniques. The estimated p-value was thresholded at 0.05 for significance. Inter-session CBF and CVR differences were computed to examine the between session variabilities in comparison to the between-subject variabilities. The repeatability was assessed by computing the within-subject CV and ICC of the estimated CBF and CVR. CV was derived as the ratio between the standard deviation of the difference between the repeated measurements to the mean of the repeated measurements. Two-way mixed single measures of ICC was calculated for the absolute agreement between repeated measures using the technique described by Mezue et al8. A low CV or high ICC value indicates high repeatability.
Results
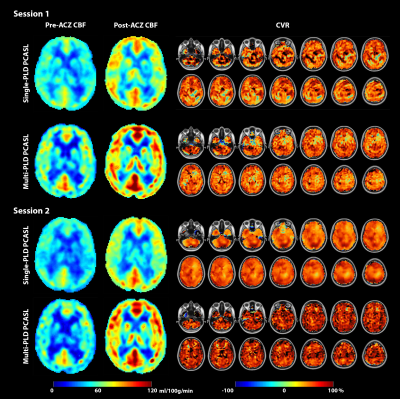
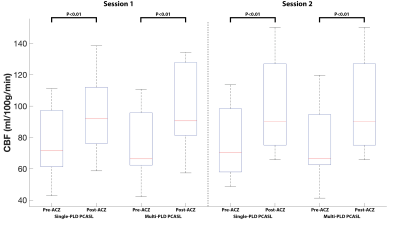
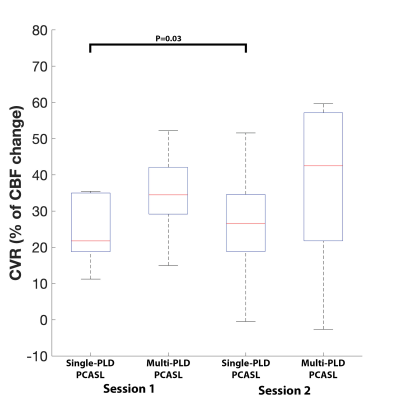
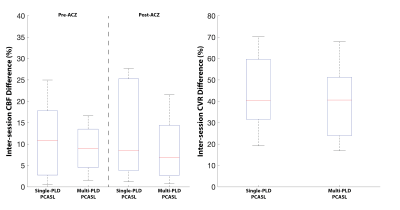
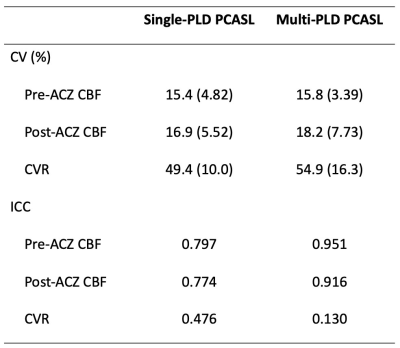
Figure 1 shows the estimated CBF and CVR of an example subject from the two sessions. Figure 2 shows the mean CBF of the whole brain. Mean CBF increased significantly post-ACZ while no significant differences were found between the two sessions. Figure 3 shows the mean CVR of both ASL techniques of the whole brain. Figure 4 shows the inter-session CBF and CVR difference. The CVR difference between the repeated measures was similar (median=40%) for both techniques. Table 1 shows the CV and ICC of CBF and CVR of the two ASL techniques. The CV of single-PLD PCASL was marginally lower (9%) than multi-PLD PCASL, implying slightly better repeatability.Discussion
The novelty of this work was to evaluate the repeatability of CVR using ASL techniques. The observed CV and ICC of the whole brain CVR were within the range reported in studies using BOLD MRI9. Comparing between the two ASL techniques, the lower CV and higher ICC of single-PLD PCASL suggested higher repeatability than that of multi-PLD PCASL. Judging by the inter-session CVR difference, however, it was difficult to separate the two ASL techniques because both achieved a similar result. The mean CVR values of this cohort were between 20% and 40%, within the range of the results reported by previous studies using ASL and ACZ as the vessel dilator4. Significant differences between the two scan sessions were found in the single-PLD PCASL data but not in the multi-PLD PCASL. This may be explained by the significant reduction in ATT post-ACZ (results not shown) that single-PLD PCASL was unable to account for. Another factor could be the variations in inversion efficiency post-ACZ. This can be corrected using flow velocity data, which is an area of ongoing investigation.Acknowledgements
This work is supported by the NIH grant R01EB025220-02 and 5K99NS102884-02.References
[1] P. Liu and J. B. De Vis, “Cerebrovascular reactivity (CVR) MRI with CO2 challenge: A technical review,” Neuroimage, vol. 187, pp. 104–115, Feb. 2019.
[2] D. C. Alsop et al., “Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia.,” Magn. Reson. Med., vol. 73, no. 1, pp. 102–116, 2015.
[3] W. W. Ni, T. Christen, J. Rosenberg, Z. Zun, M. E. Moseley, and G. Zaharchuk, “Imaging of cerebrovascular reserve and oxygenation in Moyamoya disease,” J. Cereb. Blood Flow Metab., vol. 37, no. 4, pp. 1213–1222, 2017.
[4] M. Y. Zhao et al., “Quantification of cerebral perfusion and cerebrovascular reserve using Turbo-QUASAR arterial spin labeling MRI,” Magn. Reson. Med., vol. 83, no. 2, pp. 731–748, 2020.
[5] H. J. M. M. Mutsaerts et al., “Inter-vendor reproducibility of pseudo-continuous arterial spin labeling at 3 Tesla,” PLoS One, 2014.
[6] M. A. Chappell, A. R. Groves, B. Whitcher, and M. W. Woolrich, “Variational Bayesian Inference for a Nonlinear Forward Model,” IEEE Trans. Signal Process., vol. 57, no. 1, pp. 223–236, 2009.
[7] M. W. Woolrich et al., “Bayesian analysis of neuroimaging data in FSL.,” Neuroimage, vol. 45, no. 1 Suppl, 2009.
[8] M. Mezue, A. R. Segerdahl, T. W. Okell, M. A. Chappell, M. E. Kelly, and I. Tracey, “Optimization and reliability of multiple postlabeling delay pseudo-continuous arterial spin labeling during rest and stimulus-induced functional task activation.,” J. Cereb. Blood Flow Metab., vol. 34, no. 12, pp. 1919–27, 2014.
[9] M. G. Bright and K. Murphy, “Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance,” Neuroimage, 2013.
Figures