3285
Separating spin-compartment in arterial spin labeling using delays alternating with nutation for tailored excitation (DANTE) pulse
Shota Ishida1, Hirohiko Kimura2, Naoyuki Takei3, Yasuhiro Fujiwara4, Tsuyoshi Matsuda5, Yuki Matta1, Masayuki Kanamoto1, Nobuyuki Kosaka2, and Eiji Kidoya1
1Radiological center, University of Fukui Hospital, Eiheiji, Japan, 2Department of Radiology, Faculty of Medical Sciences, University of Fukui, Eiheiji, Japan, 3Global MR Applications and Workflow, GE Healthcare Japan, Hino, Japan, 4Department of Medical Image Sciences, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan, 5Division of Ultra-high Field MRI, Institute for Biomedical Science, Iwate Medical University, Shiwa-gun, Japan
1Radiological center, University of Fukui Hospital, Eiheiji, Japan, 2Department of Radiology, Faculty of Medical Sciences, University of Fukui, Eiheiji, Japan, 3Global MR Applications and Workflow, GE Healthcare Japan, Hino, Japan, 4Department of Medical Image Sciences, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan, 5Division of Ultra-high Field MRI, Institute for Biomedical Science, Iwate Medical University, Shiwa-gun, Japan
Synopsis
Delays alternating with nutation for tailored excitation (DANTE) pulse is capable of uniform and flow velocity related signal suppression. DANTE-ASL was compared to T2-mesurement in ASL (T2-ASL) to validate what spin-compartment is suppressed by DANTE. T2-ASL showed labeled signal remained in vascular compartment even at a PLD of 2.0 s. However, ASL signals using DANTE were approximately similar value in all the PLDs (0.5, 1.0, 2.0, and 3.0 s), because ASL signals in vascular compartment were suppressed selectively and sufficiently with concurrently preserving signals in tissue compartment. We could eliminate vascular compartment from total ASL signal using DANTE.
Introduction
Intra-arterial signal is remaining labeled spins in vascular compartment at the time of imaging, leading to quantification errors of cerebral blood flow (CBF) by arterial spin labeling (ASL). Vascular suppression (VS) is used to eliminate the intra-arterial signal. Spin-compartment between vascular and tissue compartments can be separated by VS. Thus, a quantitative information on cerebral arterial blood volume (aCBV) has been provided by utilizing VS.1,2 Motion-sensitized driven-equilibrium (MSDE) has been widely used as a VS.3 Recently, it is reported that delays alternating with nutation for tailored excitation (DANTE) pulse could uniformly suppress the intra-arterial signal with static signal preservation compared to MSDE.4 In addition, DANTE is capable of flow velocity related signal suppression.5 Although there are many advantages of DANTE, it remains unknown what spin-compartment is suppressed by DANTE. On the other hand, it has been demonstrated that T2-measurement in ASL (T2-ASL) can separate spin-compartments.2,6 In the present study, we compared signal characteristics of DANTE-ASL and T2-ASL to validate what spin-compartment dealt with DANTE-ASL. We also demonstrated quantitative aCBV map by DANTE-ASL using simplified two-compartment model.7Methods
We scanned five healthy male volunteers (25.8±3.4 years old) using a 3.0 T MRI (Discovery 750, GE Healthcare). 3-delay Hadamard-encoded ASL was performed (Fig. 1a). In addition to the Hadamard-encoding section, multiple long-labeled single-delay images were acquired.8 Two types of VS, DANTE and T2-preparation were used (Fig. 1b). DANTE gradient area was 10 μs・T/m and flip angle (FADANTE) were 5°, 10°, 15°. Effective TEs of T2-preparation were 40, 80, 160 ms. T2-prepared proton density images were also obtained to calculate tissue T2-values. ASL signals and T2-values were determined in each vascular territory. Measured signals were assessed about each VS to obtain individual signal characteristics. Subsequently, the signal characteristics were compared between the two methods. We computed aCBV map from the two ASL series with and without DANTE using the simplified two-compartment model.7,9Results
Fig.2 shows T2-values at different PLDs for each vascular territory. T2-values were decreased as PLD increased. However, T2-values were larger than that of the tissue at a PLD of 2.0 s. We could not measure T2-values at a PLD of 3.0 s due to low SNR. Fig. 3 shows relation among ASL signal, PLD, and FADANTE. A larger FADANTE reduced larger ASL signal. Signal reduction rate was decreased as PLD increased. While ASL signal reduction by DANTE was observed at a PLD of 2.0 s, ASL signal was not significantly different among all the conditions at a PLD of 3.0 s. In all the PLDs, ASL signals using DANTE with 15° FA were approximately similar value. Representative DANTE-ASL images with 15° FA were shown in Fig. 4. The result of multiparametric mapping was shown in Fig. 5.Discussion
The previous T2-ASL studies reported that a greater part of the labeled spins reached the tissue compartment within 2 s.2,6 However, our results showed T2-values at a PLD of 2.0 s were larger than those of the tissues. This result indicates the labeled spins remain in the microvascular compartment, even though the PLD is 2.0 s, which has been generally considered enough time to enters the labeled spins into the tissue compartment. In addition, T2-values in the present study with various conditions were smaller than those of the reported values.2,6 We considered that it was originated from the different readout sequence from previous reports.1,2,6 Most of the previous studies used gradient-echo based sequences, which could image spins existing larger vessels compared to spin-echo based sequences.10 In contrast to gradient-echo, almost spins in larger vessels are not imaged by spin-echo sequences. Therefore, less signal contamination from the macrovascular compartment on T2-measurement may result in difference of T2-values between the current and previous studies. A larger ASL signal reduction with larger FADANTE presents DANTE efficacy can be easily manipulated by FA selection. Signal reduction rate was larger with earlier PLDs, reflecting the vascular compartment was dominant in ASL signals at early time point. ASL signal changes at a PLD of 2.0 s imply that the labeled spins still remain in the vascular compartment. This is consistent with the difference between T2 of proton density image and T2 of labeled signal at a PLD of 2.0 s. Therefore, a certain amount of labeled spins exists in the vascular compartment even at a PLD of 2.0 s. On the other hand, ASL signal was not changed among the conditions at a PLD of 3.0 s. It is considered that the greater part of the labeled spins reached the tissue compartment within 3.0 s. Smaller signal fluctuation at a PLD of 3.0 s may be derived from the nature of DANTE, because DANTE slightly reduces static spin signals.4,5 Since ASL signals in the vascular compartment were sufficiently suppressed by DANTE, ASL signals were approximately similar value at any PLDs. These results indicate that DANTE is capable of separating spin-compartments. We demonstrated the feasibility of multiparametric mapping from two series of ASL datasets, with and without VS images, using simplified two-compartment model.Conclusion
DANTE-ASL could separate the spin-compartment between vascular and tissue compartments. Homogenous aCBV map was able to obtain by DANTE-ASL.Acknowledgements
No acknowledgement found.References
- Brookes MJ, Morris PG, Gowland PA, et al. Noninvasive measurement of arterial cerebral blood volume using Look-Locker EPI and arterial spin labeling. Magn Reson Med. 2007; 58: 41-54.
- Liu P, Uh J, Lu H. Determination of spin compartment in arterial spin labeling MRI. Magn Reson Med. 2011; 65: 120-127.
- Wang J, Yarnykh VL, Hatsukami T, et al. Improved suppression of plaque-mimicking artifacts in black-blood carotid atherosclerosis imaging using a multislice motion-sensitized driven-equilibrium (MSDE) turbo spin-echo (TSE) sequence. Magn Reson Med. 2007; 58:973–981.
- Matsuda T, Kimura H, Kabasawa H, et al. Three-dimensional arterial spin labeling imaging with a DANTE preparation pulse. Magn Reson Imaging, 2018; 49: 131-7.
- Fujiwara Y, Kimura H, Ishida S, et al. Intravascular signal suppression and microvascular signal mapping using delays alternating with nutation for tailored excitation (DANTE) pulse for arterial spin labeling perfusion imaging. MAGMA, in press.
- Schmid S, Teeuwisse WM, Lu H, et al. Time-efficient determination of spin compartments by time-encoded pCASL T2-relaxation-under-spin-tagging and its application in hemodynamic characterization of the cerebral border zones. Neuroimage, 2015; 123: 72-9.
- Kimura H, Higashino Y, Ishida S, et al. ASL signal model for simultaneously measuring CBF and CBV based on ASL imaging for characterizing hemodynamic perfusion state in normal subjects and patients with moyamoya disease. Proc ISMRM, 2019; 288.
- Ishida S, Kimura H, Takei N, et al. Robust arterial transit time estimation using combined acquisition of Hadamard-encoded pCASL and long-labeled long-delay pCASL: a simulation and in vivo study. Proc ISMRM, 2019; 4946.
- Wang J, Alsop DC, Li L, et al. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn Reson Med, 2002; 48; 242-54.
- Weisskoff RM, Zuo CS, Boxerman JL, et al. Microscopic susceptibility variation and transverse relaxation: theory and experiment. Magn Reson Med. 1994; 31: 601-610.
Figures
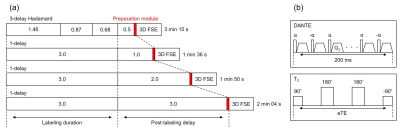
Schematic diagrams of (a) ASL acquisition design, and (b) vascular
suppression.
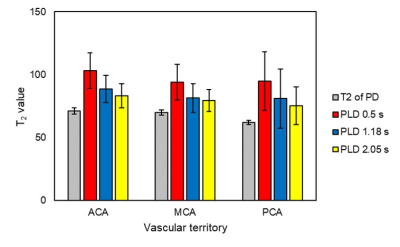
T2-values of ASL signal in each vascular territory obtained
with Hadamard-section. T2-prepared proton density images were also scanned
to determine tissue T2-values as a reference.
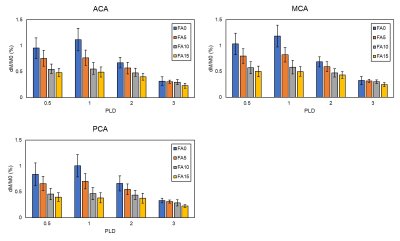
Perfusion signal at each delay time with and without DANTE using different
FADANTE. FADANTE of 0° means non-use of DANTE.
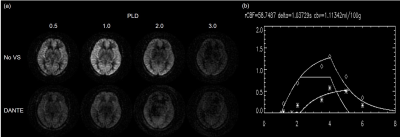
(a) Representative multi-delay perfusion images without DANTE (upper
row) and with DANTE of 15° FA (lower row), and (b) signal plot of
each ASL data and fitted lines to the simplified two-compartment model.
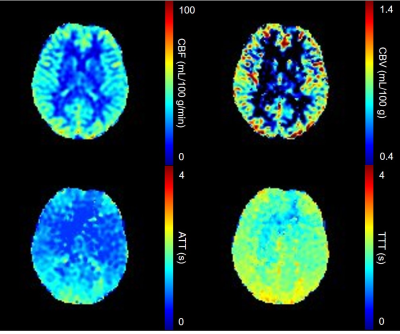
CBF, aCBV, ATT, and TTT maps computed from two
series of ASL datasets.