3277
Robust non-contrast pulmonary perfusion imaging using pseudo-continuous arterial spin labeling with background suppression1Pediatrics, UT Southwestern Medical Center, Dallas, TX, United States, 2Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 3Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Pulmonary perfusion imaging using ASL has been demonstrated using the pCASL technique with varying degrees of reproducibility. In this study, a pCASL labeling strategy is proposed to improve the stability of perfusion signal in the lungs using cardiac triggering to reduce flow-induced signal variations, and background suppression to improve SNR. With the proposed technique, high SNR perfusion images are created with a limited number of signal averages. This optimized approach may allow full coverage of the lungs in clinically reasonable scan times.
Introduction
Non-contrast perfusion imaging using arterial spin labeled (ASL) MRI has been well established in the lungs using the pulsed-ASL FAIR technique1,2. However, pseudo-continuous ASL (pCASL) has been shown to offer improved SNR, and is recommended for brain perfusion imaging3. The extension of pCASL to study pulmonary perfusion has been non-trivial due to the highly pulsatile blood flow and complex anatomy of the vasculature, posing a significant challenge in identifying the vessel of interest for successful labeling.pCASL has been previously demonstrated for pulmonary perfusion imaging4-6. However, inconsistent results were achieved due to the extended pCASL repetition time rendering the acquisition sensitive to cardiac phase5. Cardiac triggering the acquisition following the post-label delay can ensure consistent diastolic acquisitions, but the resulting variable post-label delay is not compatible with background suppression, which has been shown to significantly improve ASL perfusion images3.
Therefore, the purpose of this study was to implement a pCASL labeling technique to improve the stability of the ASL signal in the lungs using an effective cardiac triggering strategy that is compatible with background suppression and allows perfusion quantification.
Methods
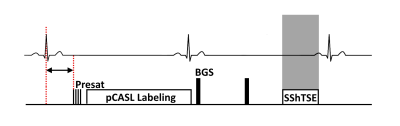
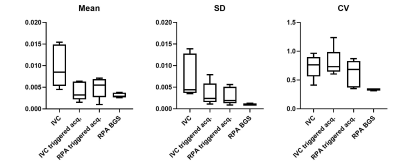
This was a HIPAA-compliant study approved by the institutional review board. All subjects provided written informed consent prior to the participation in the study. Figure 1 shows the proposed pCASL sequence that was implemented on a 3T Ingenia scanner (Philips Healthcare, Best, The Netherlands). The saturation pulses at the start of the sequence were cardiac triggered4, and the label duration (LD) and post-label delay (PLD) were reduced compared to timings used in the brain and kidneys3,7, such that the acquisition occurs during the diastolic period of the following heartbeat, and were set to 600-750ms depending on the subjects heartrate. This results in consistent diastolic acquisitions to reduce signal variations introduced by pulsatile flow1,5. Two background suppression pulses were applied during the PLD to improve SNR and reduce physiological noise, and a single-shot turbo spin echo (SShTSE) acquisition was used to reduce susceptibility artifacts in the lungs. A guided breathing approach was used to reduce motion during the acquisition, and 4-8 signal averages were acquired in 0:48 - 1:30 minutes. 2D FAIR images were acquired in each subject for comparison, and a proton-density (M0) image was acquired for perfusion quantification8.The proposed labeling and cardiac triggering strategy was evaluated in 5 healthy volunteers. These images were compared with previously presented data from an additional 15 volunteers, who were scanned with various approaches applying pCASL in the lungs, which were progressively modified to improve the stability of the perfusion signal. These approaches include labeling of the IVC with the start of the sequence cardiac triggered, IVC labeling with a cardiac triggered acquisition for more consistent cardiac phase, and RPA labeling with a cardiac triggered acquisition to reduce the transit time of labeled blood. The coefficient of variation of the perfusion signal across dynamics was used to evaluate the signal stability of each of these approaches.
Results
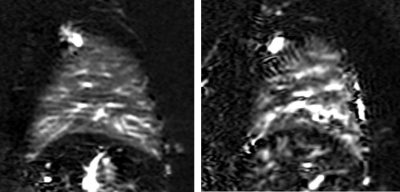
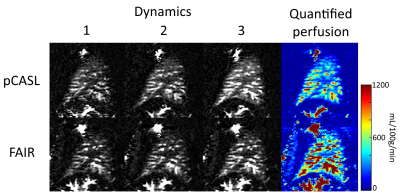
The proposed scheme with cardiac triggering and background suppression provided robust pulmonary perfusion-weighted images with only 3 signal averages (Fig. 2). This approach also provided consistent perfusion-weighted images across different dynamics, that are comparable to FAIR (Fig. 3). Additionally, pCASL images demonstrated reduced vascular signal, since the inflowing perfusion bolus has a fixed duration, unlike FAIR9.The average coefficient of variation across dynamics was 0.74 ± 0.18 (average ± SD) for the IVC labeling approach, 0.80 ± 0.22 for IVC labeling with the cardiac triggered acquisition, 0.62 ± 0.21 for RPA labeling with the cardiac triggered acquisition, and 0.34 ± 0.02 for RPA labeling with the shorter TR and background suppression, indicating that the proposed approach provides the most stable perfusion signal.
The average perfusion in the five subjects scanned with the proposed RPA labeling with background suppression technique was 435 ± 65 mL/100g/min, within the normal expected range2.
Discussion
Previous attempts at generating pulmonary perfusion maps with pCASL have provided promising, yet inconsistent results due to variations in cardiac phase of the acquisition, limited SNR, and extended scan times. The proposed pCASL labeling and cardiac triggering strategy addressed these limitations and provided consistent perfusion signal in the lungs. The improved SNR with this technique allows fewer signal averages to be acquired and should allow full coverage of the lungs to be achieved in clinically reasonable scan times for complete assessment of pulmonary perfusion.Acknowledgements
No acknowledgement found.References
1 Greer, J. S. et al. Non-contrast quantitative pulmonary perfusion using flow alternating inversion recovery at 3 T: A preliminary study. Magnetic resonance imaging 46, 106-113 (2018).
2 Bolar, D. S. et al. Quantification of regional pulmonary blood flow using ASL-FAIRER. Magn Reson Med 55, 1308-1317, doi:10.1002/mrm.20891 (2006).
3 Alsop, D. C. et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. 73, 102-116 (2015).
4 Martirosian, P. et al. Measurement of Lung Perfusion Using Optimized Pseudo-Continuous Arterial Spin Labeling of Pulmonary Arteries and Fast True-FISP Imaging at 3 Tesla. International Society for Magnetic Resonance in Medicine (2017).
5 Joshua S. Greer, Xinzeng Wang, Ivan Pedrosa & Madhuranthakam, A. J. Pulmonary Perfusion using pseudo-continuous Arterial Spin Labeling. International Society for Magnetic Resonance in Medicine (2016).
6 Petros Martirosian et al. Measurement of Pulmonary Perfusion using PCASL True-FISP Imaging at 1.5 Tesla. International Society for Magnetic Resonance in Medicine (2018).
7 Robson, P. M. et al. Volumetric arterial spin-labeled perfusion imaging of the kidneys with a three-dimensional fast spin echo acquisition. Academic radiology 23, 144-154 (2016).
8 Buxton, R. B. et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. 40, 383-396 (1998).
9 Wong, E. C., Buxton, R. B. & Frank, L. R. J. M. r. i. m. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). 39, 702-708 (1998).
Figures