3276
Off-resonance and flow-velocity immune ASL at low-power using pseudo-continuous saturation labeling1Division of MRI research, Department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
Synopsis
While widely used for perfusion imaging, the flow-driven inversion labeling commonly used with pseudo-continuous ASL (PCASL) presents a significant off-resonance sensitivity as well as high power deposition potentially limiting high-field applications. We therefore investigated an alternate labeling scheme that takes advantage of the multiple aliased labeling planes that arise within the labeling RF envelope when reducing the peak-to-average gradient ratio. First numerical simulations and experiments show the potential for low-SAR, B0 and flow-velocity robust saturation labeling.
Introduction
Since its inception, continuous flow-driven adiabatic inversion using pulsed RF and gradients, known as PCASL1, has become a popular method for Arterial Spin Labeling (ASL) perfusion2 because of higher efficiency compared to continuous ASL3, multi-slice approaches but also theoretically higher SNR compared to pulsed methods4,5. Nonetheless, the off-resonance sensitivity as well as high power deposition hamper PCASL applications at ultra-high-field (e.g.≥7T). While saturation-based labeling techniques have been among the first proposed for ASL6,7, their use has been limited because of their lower SNR compared to inversion strategies and the lack of an effective control strategy to compensate for direct MT and other effects on imaged tissue. We report here the implementation and early optimization of a pseudo-continuous saturation based labeling strategy for low SAR robust perfusion imaging.Material and Methods
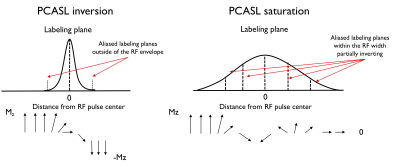
TheoryWhen PCASL is optimized for inversion labeling, the gradients and RF pulses are designed to create one inversion plane within the RF pulse width while aliased inversion planes are outside the pulse width. Off-resonance sensitivity arises in PCASL inversion because it induces shifts of the labeling plane away from the center of the pulse where RF power is lower. Conversely, for saturation labeling, we reduce the ratio of the RF slice selection gradient to the time averaged gradient such that multiple aliased labeling planes are within the slice profile. This introduces multiple, partially efficient “inversions” that ultimately lead to saturation of flowing spins in a manner similar to DANTE flow saturation8. Off-resonance sensitivity is greatly reduced relative to inversion because it merely shifts the multiple lines around within the pulse envelope (Fig.1). The unbalanced gradient control used in PCASL inversion also serves as an effective control for saturation.
Simulations
We simulated labeling efficiency using a numerical integration of Bloch equations as described previously1,9,10. We simulated efficiency over a range of average power (B1av) from 0.2 to 1.5μT and peak-to-average gradient ratio from R=1 to 7 by 0.5 steps with an average gradient set at 0.5mT/m (to avoid being close to background gradients levels) at a fixed flow-velocity of 50cm/s for a Hann-shaped pulse of 500μs played every 1ms or shorter when hardware compatible.
Experiments
Three volunteers were scanned at 3T (GE Discovery MR750) using 32-ch coils for brain (N=1) and kidney (N=2) imaging. A single-slice single-shot FSE was acquired, positioned axial mid-ventricles for the brain and mid-kidneys coronal. SSFSE parameters were TR/TE=6000/40ms,mtx=1282,rBW=20.83kHz, 7-mm thick slice, flip-angle=120°. We acquired two datasets in each case with either the recommended optimized inversion scheme (B1av=1.4μT,R=7,Gav=0.5mT/m)10 and the proposed saturation scheme (B1av=0.75μT,R=2,Gav=0.5mT/m) with a background-suppressed (BS) PCASL preparation using a w=1.5s labeling and a single PLD=1.5s (kidney) and 2s (brain), with 14 pairs for the brain and 7 for the kidneys. Kidney acquisitions were acquired with a timed-breathing strategy.
Quantification
Image reconstruction was performed offline, with a complex ASL subtraction followed by homodyne reconstruction. We quantified absolute cerebral and renal blood-flows (f) using a 1-compartment model11:
$$f=[6000\cdot\lambda\cdot dM\cdot \exp(PLD/T_{1b})]/[IF\cdot \alpha\cdot T_{1b} \cdot M_{0} \cdot \exp(-w/T_{1b})]$$ With IF=1 for the saturation-based and 2 for the inversion-based labeling strategies. The labeling efficiency a was estimated at 0.6 for PCASL-I (0.8x0.75 for BS) and 0.8 for PCASL-S because of BS. We calculated a mean SNR for both labeling schemes and a ratio of PCASL-I/PCASL-S.
Results
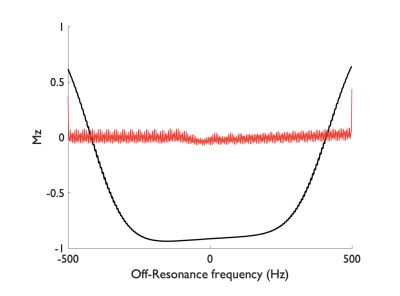
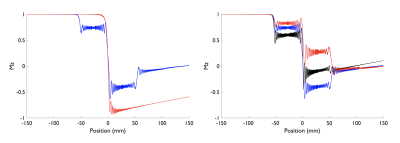
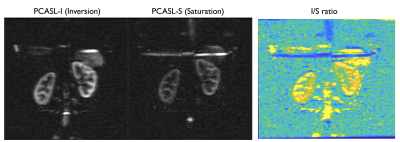
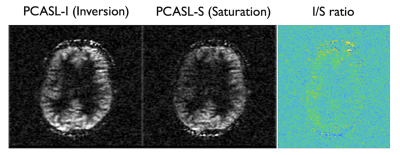
Simulation results show that by reducing the average B1 (0.75μT) with a low gradient ratio (2), it is possible to obtain a flat efficiency of 0 corresponding to spins saturation over a wide-range of off-resonance frequencies (Fig.2) compared to optimized PCASL inversion labeling. When looking at the spatial profile of longitudinal magnetization, we see that, in accordance with theory, multiple labeling planes appear within the Hann-pulse envelope eventually leading to spins saturation, but also that this strategy is somewhat robust across a range of flow velocities (Fig.3).First imaging experiments in kidneys (Fig.4) and brain (Fig.5) are encouraging. As expected, we measured a lower SNR using the PCASL-S vs I (21.0vs36.9) in the kidney scan but the difference in SNR was not marked in the brain (16vs15). We observed a mild reduction in ASL signal fluctuation across repetitions from 6 to 4% in the brain (STD% of the mean perfusion signal) but more significant in the kidney case from 26 to 18%, suggesting increased labeling temporal stability. Additionally, we noted a marked reduction of SAR from 2 to 1.1W/kg for the kidney and 1.7 to 0.9W/kg for the brain. Finally, for quantification, we observed numbers consistent with healthy renal flow while markedly reducing in-ROI STD in the renal cortex in the saturation case (261±80 vs 224±40mL/100/min). In the brain, similar observations were made (80±20 vs 61±15mL/100g/min).
Discussion and conclusions
Those preliminary theoretical optimizations and experiments show that saturation-based labeling strategies based on a minor modification of PCASL can lead to an off-resonance robust labeling while greatly reducing power deposition, potentially paving the way for ultra-high-field robust ASL perfusion imaging. While somewhat reducing SNR, this saturation labeling also facilitates ASL quantification by reducing the influence of labeling efficiency on estimated blood-flow by rendering it much more insensitive to off-resonance. However, more work is required to fully characterize labeling efficiency and effects on SNR to provide optimal flow-velocity and B0/B1 robustness.Acknowledgements
No acknowledgement found.References
1. Dai, W., Garcia, D., de Bazelaire, C. & Alsop, D. C. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn. Reson. Med. 60, 1488–1497 (2008).
2. Alsop, D. C. et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 73, 102–116 (2015).
3. Alsop, D. C. & Detre, J. A. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology 208, 410–416 (1998).
4. Wong, E. C., Buxton, R. B. & Frank, L. R. A theoretical and experimental comparison of continuous and pulsed arterial spin labeling techniques for quantitative perfusion imaging. Magn. Reson. Med. 40, 348–355 (1998).
5. Wang, J. et al. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn. Reson. Med. 48, 242–254 (2002).
6. Detre, J. A., Leigh, J. S., Williams, D. S. & Koretsky, A. P. Perfusion imaging. Magn. Reson. Med. 23, 37–45 (1992).
7. Francis, S. T., Gowland, P. A. & Bowtell, R. W. Continuous saturation EPI with diffusion weighting at 3.0 T. NMR Biomed. 12, 440–450 (1999).
8. Li, L., Miller, K. L. & Jezzard, P. DANTE-prepared pulse trains: A novel approach to motion-sensitized and motion-suppressed quantitative magnetic resonance imaging. Magn. Reson. Med. 68, 1423–1438 (2012).
9. Maccotta, L., Detre, J. A. & Alsop, D. C. The efficiency of adiabatic inversion for perfusion imaging by arterial spin labeling. NMR Biomed. 10, 216–221 (1997).
10. Zhao, L., Vidorreta, M., Soman, S., Detre, J. A. & Alsop, D. C. Improving the robustness of pseudo-continuous arterial spin labeling to off-resonance and pulsatile flow velocity. Magn. Reson. Med. (2016) doi:10.1002/mrm.26513.
11. Alsop, D. C. & Detre, J. A. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 16, 1236–1249 (1996).
Figures