3273
Reliability and Reproducibility of ASL-MRI Measured Perfusion in GBM at 3T1Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Harold C. Simmons Comprehensive Cancer Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 4Neurological Surgery, University of Texas Southwestern Medical Center, Dallas, TX, United States, 5Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, TX, United States, 6Population and Data Sciences, University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
We have assessed the reliability of pCASL using 3D TSE-CASPR in healthy volunteers and glioblastoma (GBM) patients. “Excellent” agreement was observed for intrasession reliability in both normal appearing grey matter (NAGM) and tumors with intraclass correlation coefficient (ICC) of 0.945 (95% CI: 0.916-0.964) and 0.948 (95% CI: 0.883-0.978) respectively. Across multiple ROIs, the within-subject coefficient of variation (wsCV) was measured to be 6.1 ± 1.2% across all subjects in NAGM, and 5.1% in tumors. The lower wsCV in tumors allow 3D TSE pCASL using CASPR to be used for longitudinal monitoring of GBM patients.
Introduction
Arterial spin labeled (ASL) MRI has emerged as a promising method to measure non-contrast perfusion in the brain. Several groups have shown good reliability and reproducibility of ASL-MRI measured brain perfusion in healthy volunteers. Almost all of these techniques used EPI1, GRASE2 or spiral based TSE3 acquisition. For application in glioblastoma (GBM) patients, who often have craniotomy, these acquisitions may suffer from B0 inhomogeneities. Recently, a 3D TSE using Cartesian acquisition with spiral profile reordering (CASPR) in combination with pseudo-continuous ASL (pCASL)4 was developed to improve the robustness of ASL measured perfusion. In this study, we evaluated the reliability and reproducibility of 3D TSE with CASPR measured brain perfusion in both healthy volunteers and GBM patients at 3T.Methods
Subjects: Under IRB approved protocols, 2 healthy volunteers and 5 newly diagnosed GBM patients with a mean age of 46 ± 19 years were recruited and underwent 10 imaging sessions on a 3T MRI scanner (Ingenia, Philips Healthcare).Imaging acquisition and post-analysis: MRI scans were acquired with 32-channel head coil. In each imaging session, routine clinical protocol for GBM patients and two repetitions of 3D TSE-CASPR pCASL were performed for each subject. The protocol began with 3D T1W MPRAGE, followed by the first run of 3D TSE-CASPR pCASL, T2 FLAIR, SWI and the second run of 3D TSE-CASPR pCASL. ASL scans were acquired in the axial plane with the following parameters (according to the ASL consensus paper5): TR/TE = 6000/14 ms, FOV = 220x220x110 mm3, matrix = 64x64 with 36 slices, acquired resolution = 3.5x3.5x6 mm3, reconstructed resolution = 3x3x3 mm3, echo spacing = 2.8 ms, ETL = 80, label duration = 1.8 s, post-label delay = 1.8 s, 1 repetition, 4 background suppression pulses and acquisition time = 3:00 minutes. A M0 image was acquired using same acquisition parameters in 1:30 minutes. For comparison, ASL images were also acquired using the vendor supplied 3D GRASE acquisition in all subjects matching the same acquisition parameters as 3D TSE-CASPR except for: TR/TE = 3900/14 ms, signal averages = 3, and total acquisition time = 4:30 mins, including a M0 acquisition, matching the total acquisition time of 3D TSE-CASPR.
3D TSE-CASPR pCASL images were reconstructed on the scanner including k-space filtering and complex k-space subtraction6. Perfusion difference images from 3D TSE-CASPR were transformed into NIfTI with MRIcron software followed by FSL for brain extraction. The NIfTI images were processed to quantify CBF maps in MATLAB based on the ASL consensus paper5. 3D GRASE pCASL images were processed into CBF maps on the scanner using vendor supplied reconstruction. ROIs were drawn for normal appearing grey matter (NAGM) in several locations and tumors and the mean ± standard deviation of CBF values in ml/100g /min were tabulated.
Statistical analysis: The reliability between the two runs of 3D TSE-CASPR pCASL measurements were measured using intraclass correlation coefficient (ICC) and Bland-Altman plots. ICC estimates and their 95% confident intervals (CI) were calculated using SPSS statistical package version 24.0 (SPSS Inc, Chicago, IL) based on a single-measurement, absolute-agreement, 2-way mixed-effects model. Within-subject coefficients of variation (wsCV), defined as the ratio of the standard deviation (SD) of the difference between repeated measurements to the mean of the repeated measurements was also measured.
Results
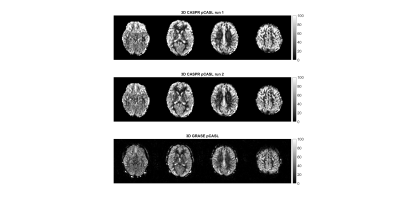
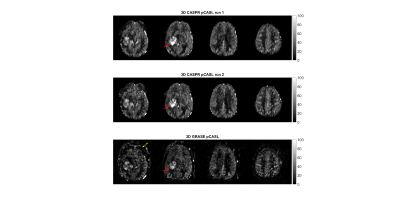
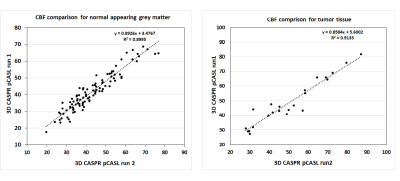
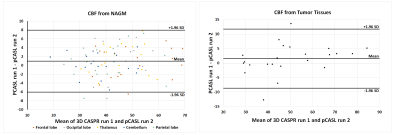
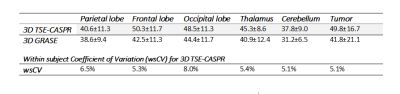
3D TSE-CASPR achieved robust CBF maps in all subjects with similar values in healthy volunteers (Fig. 1), and in GBM patients (Fig. 2). Compared to 3D GRASE, the CBF maps with 3D TSE-CASPR had less image distortion in GBM patients (Fig. 2, dashed arrow). The correlation plots (Fig. 3) and Bland-Altman plots (Fig. 4) together with 95% CI of the CBF values for NAGM and tumors showed good reliability of 3D TSE-CASPR. The ICC for NAGM was 0.945 (95% CI: 0.916-0.964) and the estimated ICC for tumors was 0.948 (95% CI: 0.883-0.978). The mean CBF values measured with 3D TSE-CASPR were slightly higher than the 3D GRASE values (Table 1). The wsCVs across several brain ROIs was 6.1% ± 1.2%, and 5.1% in tumors across all subjects (Table 1).Discussion and Conclusion
The 3D TSE-CASPR provided robust CBF maps in both healthy volunteers and GBM patients with high intrasession repeatability at 3T, indicating it will be an appropriate non-contrast and non-invasive method for longitudinal GBM treatment response management. The CBF values from 3D TSE-CASPR were also consistent with the literature values and were slightly higher than 3D GRASE, and needs further evaluation.Acknowledgements
This work was partly supported by the NIH/NCI grant U01CA207091.References
1. Chen, Y et al., JMRI, 2011; 33(4): 940–949.
2. Kilroy, E et al., JMRI, 2014; 39(4): 931-939.
3. Musaerts, HJMM et al., PLoS ONE 2014; 9(8): e104108.
4. Greer, J. S., et al., MRM, 2019. 82(5): p.1713-1724.
5. Alsop, D.C., et al., MRM, 2015. 73(1): p. 102-16.
6. Wang, Y et al., ISMRM 2019:4962.
Figures