3272
DW-pCASL measure of BBB water permeability is sensitive to pharmacological manipulation of AQP4 function1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States, 2USC Stevens Neuroimaging and Informatics Institute, University of Southern California, Los Angeles, CA, United States
Synopsis
The blood-brain barrier (BBB) is a tightly regulated structure that protect the central nervous system (CNS). BBB impairment is implicated in several brain disorders, such as Alzheimer’s disease. Aquaporin-4 (AQP4) water channels are critically involved in regulating brain water transport across the BBB. We have recently developed a diffusion-weighted pCASL (DW-pCASL) technique allowing measurement of water permeability across the BBB. In this study, we implemented and optimized DW-pCASL in nonhuman primates. We directly validated that the underlying signal mechanism of DW-pCASL is related to AQP4-mediated BBB water exchange using a pharmacological challenge.
Introduction
The blood-brain barrier (BBB) is a tightly regulated structure that prevents the entry of potentially harmful molecules to the brain and facilitates the transport of water, nutrients, and waste. Dysfunction of BBB is associated with a number of CNS disorders including multiple sclerosis, stroke, brain tumors, and Alzheimer’s disease. Aquaporin-4 (AQP4) water channels are critically involved in regulating brain water transport and maintaining homeostasis. Accumulating evidence also supports the role of AQP4 in mediating water-dependent clearance of toxic proteins, such as amyloid-beta, from the brain. Recently, we have developed a 3D diffusion-weighted pseudo-continuous ASL (DW-pCASL) allowing quantification of BBB water permeability with good reproducibility1. In this study, we aimed to implement and optimize the DW-pCASL sequence for use in large nonhuman primates (NHPs). More importantly, we used a pharmacological challenge to directly manipulate AQP4 function in order to validate the underlying signal mechanism of DW-pCASL and to assess the sensitivity of BBB water permeability to changes in AQP4 function.Methods
MRI images were acquired on four macaques (male, ~6-12 kg) using a 3T Siemens mMR and an 8-channel coil. Animals were anesthetized with isoflurane (1.0%) and the physiological parameters were maintained within normal ranges. Experiment 1. Protocol optimization: A full set of DW-pCASL data at multiple PLDs (800, 1100, 1400, and 1700 ms) with five b-values from 0 to 120 s/mm2 (0, 10, 25, 50, 100, 120 s/mm2) were acquired from all the NHPs (N=5 on 4 NHPs) in order to determine the optimal imaging parameters. Experiment 2. Pharmacological challenge: two PLDs = 600 and 1100 with b values of 0/10 and 0/25 s/mm2 were chosen as the short two-stage protocol for pharmacological experiments in order to estimate arterial transit time (ATT) and water exchange rate (kw) across the BBB2, respectively. Two doses of an AQP4 inhibitor, TGN-020 (1.0 and 1.8 mg/kg), were given intravenously to two NHPs3. DW-pCASL data were acquired before and after the drug challenge. Data were analyzed with custom code written in matlab as described previously1. In short, a single-pass approximation (SPA) model2 was used for mapping ATT and kw with total generalized variation (TGV) regularization4. Percent changes of ATT and kw before and after AQP4 challenge were also calculated.Results
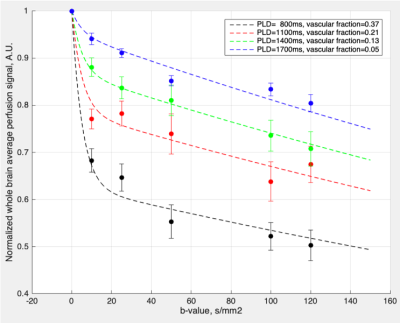
Average perfusion signals with multiple PLD and b-values from four NHPs (N=5; marks) and bi-exponential fitting results (curves) are shown in Figure 1. Biexponential fitting results are shown in the upper right corner. On average, 63%, 79%, 87% and 95% of labeled blood enters brain tissue space at the PLD of 800, 1100, 1400, and 1700 ms, respectively. Estimated (pseudo‐)diffusion coefficients of capillary/brain tissue were 0.19/0.002 mm2/s when simultaneously fitting of 4 PLDs. Based on these results, b = 10 s/mm2 and PLD = 600 ms were chosen to estimate ATT. For kw measurements in the subsequent pharmacological experiment, b = 25 s/mm2 and PLD = 1100 ms were chosen. The mean whole brain CBF, ATT and kw were 34.4 ± 12.9 ml/100g/min, 974.6 ± 71.7 ms, 171.0 ± 42.4 min-1, respectively. AQP4 inhibition with TGN-020 did not induce significant changes in ATT (N=2). A dose of 1.0 mg/kg of TGN-020 caused a ~14.2% reduction in kw (N=1, from 219 to 188 min-1; Figure 2) while a dose of 1.8 mg/kg of TGN-020 resulted in a ~27.1% reduction in kw (N=1, from 162 to 118 min-1).Discussion
We have optimized the DW-pCASL in large NHPs and demonstrated the feasibility of mapping kw in the NHP brain. The average DW-pCASL signals at multiple PLDs and b-values from four NHPs are highly consistent with those acquired in humans, demonstrating more water exchanging into the slow-decaying or tissue compartment with longer PLDs. Nevertheless, the ATT is shorter in NHPs than humans. The b-value for differentiating the slow and fast-decaying components in NHPs is lower than that in human subjects, emphasizing the importance of tailored parameters for different species. Our preliminary results demonstrate a dose-dependent reduction of kw in response to AQP4 inhibitor challenges as expected, suggesting that DW-pCASL can be applied to estimate AQP4-mediating water exchange across the BBB. Our result is consistent with a recent multi-delay multi-echo ASL study in AQP4 knock-out mice5. In addition, our results also support the use of BBB water permeability measurements (kw or permeability-surface product of water (PSW) measured at longer PLD) as a surrogate for AQP4 function. Study is currently on-going to include additional doses of pharmacological challenges.Conclusions
We have directly validated that the underlying signal mechanism of DW-pCASL is related to AQP4-mediated BBB water exchange using a pharmacological challenge in NHPs. Our results support the use of BBB water permeability as a surrogate for AQP4 function, which has high clinical relevance to better understanding AQP4-related clearance function in neurodegenerative disorders. DW-pCASL may also be used in future studies to estimate BBB water permeability in NHP models of brain disorders or to monitor the response of novel AQP4-targeting medication.Acknowledgements
No acknowledgement found.References
1. Shao X, Ma SJ, Casey M, D'Orazio L, Ringman JM, Wang DJJ. Mapping water exchange across the blood-brain barrier using 3D diffusion-prepared arterial spin labeled perfusion MRI. Magn Reson Med. 2019;81(5):3065-3079.
2. St Lawrence KS, Owen D, Wang DJ. A two-stage approach for measuring vascular water exchange and arterial transit time by diffusion-weighted perfusion MRI. Magn Reson Med. 2012;67(5):1275-1284.
3. Suzuki Y, Nakamura Y, Yamada K, Huber VJ, Tsujita M, Nakada T. Aquaporin-4 positron emission tomography imaging of the human brain: first report. J Neuroimaging. 2013;23(2):219-223.
4. Knoll F, Bredies K, Pock T, Stollberger R. Second order total generalized variation (TGV) for MRI [published online ahead of print 2011/01/26]. Magn Reson Med. 2011;65(2):480-491.
5. Ohene Y, Harrison IF, Nahavandi P, et al. Non-invasive MRI of brain clearance pathways using multiple echo time arterial spin labelling: an aquaporin-4 study. In: Neuroimage. Vol 188.2019:515-523.
Figures