3266
Time Course of Cerebral Blood Flow in Patients with Symptomatic Middle Cerebral Artery Stenosis during Lipid-lowering Treatment1Tsinghua University, Beijing, China
Synopsis
Statin treatment is considered as an effective method to stabilize atherosclerosis and potentially improve cerebral perfusion. This study evaluated the changes of artery stenosis and CBF on arterial spin labeling during statin treatment with two-years’ follow-up. We found that CBF improved in the symptomatic territory among all subjects at 6th month (rCBF: 0.92±0.07 vs. 0.95±0.07, P=0.001). After 6 months, however, CBF decreased in patients with stenosis progression whereas maintained at a stable level in patients with stenosis regression, suggesting that changes of symptomatic MCA stenosis may have an effect on the cerebral hemodynamic improvements during statin treatment in different stages.
Introduction
Intracranial artery stenosis, especially the middle cerebral artery (MCA) stenosis, is the main cause of ischemic cerebrovascular disease and strong predictor for recurrent events.1 It has been shown that statins are reductase inhibitors that delay the progression of atherosclerosis, stabilize atherosclerotic plaques, and even reduce the severity of atherosclerosis2. The other potential benefit of statin therapy might be the improvement of cerebral perfusion to maintain normal nerve function and metabolism activity of brain tissue by reducing arterial luminal stenosis. However, the time course of cerebral blood flow (CBF) along with the changes of arterial stenosis in symptomatic patients during statin treatment is unknown. In this study, we aimed to investigate the changes of CBF along with the changes of symptomatic intracranial arterial stenosis during the lipid-lowering treatment using high-resolution vessel wall imaging (HR-VWI) and arterial spin labeling (ASL).Methods
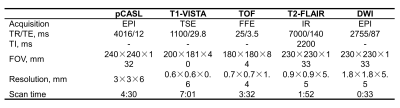
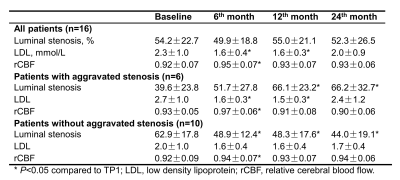
Study sample: A total of 16 patients (mean age, 59.1 ± 7.9 years; 10 males) with symptomatic unilateral middle cerebral artery stenosis (30%-79%) were enrolled in this study. All the patients received two years’ resuvastatin treatment with 10-20 mg/d and were followed-up with MRI examinations. MR imaging: After written informed consent was obtained, all subjects underwent MR imaging on a 3T MR scanner (Achieva TX, Philips Healthcare, Best, The Netherlands) equipped with a 32-channel head coil. The MR imaging protocol included pCASL, T1-VISTA (before and after gadolinium-enhancement), TOF, T2-FLAIR and DWI sequences and the imaging parameters were summarized in Table 1. The MR imaging was performed at baseline, 6, 12 and 24 months after treatment. Image analysis: The luminal stenosis was measured on the T1-VISTA vessel wall images using WASID criteria by two experienced radiologists blinded to clinical information and time point with consensus.3 The changes of intracranial artery stenosis at the follow-up time point against baseline were measured. The CBF was calculated using the pCASL images with proton density-weighted images after motion correction.4 Then, CBF maps were co-registered to the T2-FLAIR sequence and spatially normalized to the standard Montreal Neurological Institute template space using SPM12 toolbox (Wellcome Trust, England). The volumetric MCA territories were extracted from the automated anatomic labeling template based on gray matter mask and excluded from the infarct regions observed on T2-FLAIR.5 Relative CBF (rCBF) was therefore defined as the mean values on the ischemic side divided by the corresponding values on the contralateral side. Statistical analysis: The statistical analysis was performed using the software of SPSS 16.0 (IBM, Chicago, IL). The continuous variables were described as mean and standard deviation (SD). Paired t-test was used to determine the difference in lipoprotein levels, intracranial artery stenosis, and rCBF between the last three time points (6, 12 and 24 months after treatment) and baseline (0 month). Two-tailed P-values less than 0.05 were considered statistically significant.Results
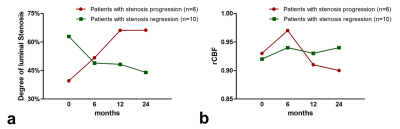
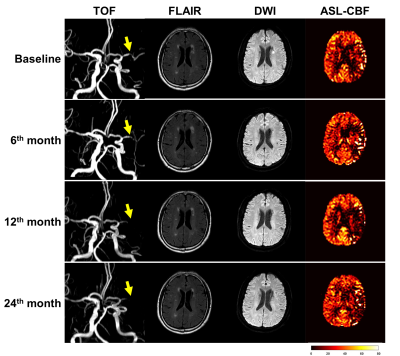
Of all 16 patients enrolled in this study, 6 (37.5%) and 10 (62.5%) had progression and regression in the luminal stenosis after two years’ resuvastatin treatment (Table 2), respectively. Improvements of CBF in the symptomatic territory were found among all subjects at 6th month during statin treatment (rCBF: 0.92±0.07 vs. 0.95±0.07, P=0.001), along with the decline of low-density lipoprotein (LDL) (2.3±1.0 mmol/L vs. 1.6±0.4 mmol/L, P=0.014). However, CBF showed decreasing trend in patients with stenosis progression whereas CBF maintained at a stable level in patients with stenosis regression after 6 months of statin treatment (Figure 1). Figure 2 shows representative examples from aggravated group after resuvastatin treatment.Discussion and Conclusions
In this study population, our results showed that the resuvastatin treatment for symptomatic MCA stenosis would cause various outcomes of perfusion improvement in the different stages. CBF was improved to some extent on the symptomatic territories at the sixth month, which was consistent with the significant cholesterol lowering. Subsequently, the degree of vascular stenosis affected the alterations of cerebral perfusion. For patients who benefited from the drug therapy, their brains regained the relatively good and stable blood supply which may be contributable to the stabilization of vulnerable plaques. For patients who had poor therapeutic effect, more severe luminal stenosis led to the further hemodynamic destruction. Both factors may induce the cerebral infarction, causing worse chain reaction.6 Under the circumstances, alternative treatment strategy, such as intensive statin therapy7, needs to be considered which needs further research. In summary, changes of symptomatic MCA stenosis may have an effect on the cerebral hemodynamic improvements during statin treatment in different stages.Acknowledgements
No acknowledgement found.References
1. WASID Trial Investigators. Design, progress and challenges of a double-blind trial of warfarin versus aspirin for symptomatic intracranial arterial stenosis. Neuroepidemiology. 2003; 22(2): 106-117.
2. Ni Chroinin D, Asplund K, Asberg S, et al. Statin therapy and outcome after ischemic stroke: systematic review and meta-analysis of observational studies and randomized trials. Stroke. 2013; 44(2): 448-456.
3. Zhao H, Wang J, Liu X, et al. Assessment of carotid artery atherosclerotic disease by using three-dimensional fast black-blood MR imaging: comparison with DSA. Radiology. 2015; 274(2): 508-516.
4. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1): 102-116.
5. Lyu J, Ma N, Liebeskind DS, et al. Arterial Spin Labeling Magnetic Resonance Imaging Estimation of Antegrade and Collateral Flow in Unilateral Middle Cerebral Artery Stenosis. Stroke. 2016; 47(2): 428-433.
6. Kashiwazaki D, Akioka N, Kuwayama N, et al. Pathophysiology of acute cerebrovascular syndrome in patients with carotid artery stenosis: a magnetic resonance imaging/single-photon emission computed tomography study. Neurosurgery. 2015; 76(4): 427-433.
7. Zhou P, Lu Z, Gao P, et al. Efficacy and safety of intensive statin therapy in Chinese patients with atherosclerotic intracranial arterial stenosis: a single-center, randomized, single-blind, parallel-group study with one-year follow-up. Clin Neurol Neurosurg. 2014; 120: 6-13.
Figures