3255
Shielded Electromagnetic Transducer for Liver Magnetic Resonance Elastography1Electrical and Computer Engineering, University of British Columbia, Vancouver, BC, Canada, 2UBC MRI Research Center, Vancouver, BC, Canada, 3Department of Radiology, University of British Columbia, Vancouver, BC, Canada
Synopsis
Electromagnetic shielded Lorentz coil actuator allows using the magnetic field of the MRI scanner to induce arbitrary elastic waves in the tissue in a cost-effective way. We developed a Lorentz coil actuator for the liver magnetic resonance elastography (MRE) for providing a larger wave coverage area, where the Lorentz coil is placed outside the imaging area by the use of an additional end-effector. Initial testing for phantom showed an excellent agreement with the reported elasticity, while the liver elasticity study provided promising results in detecting cirrhosis and hepatitis C virus.
Introduction
Magnetic resonance elastography1 captures the displacement induced by external actuators to image the tissue stiffness and viscosity, which have shown to change due to abnormality in the liver, brain, and prostate2. A Lorentz coil actuator that utilizes the magnetic field of the MR scanner provides a cost-efficient, platform-independent solution for a transducer with low hardware requirements3. However, in the liver, where the Lorentz coil actuator has been usually placed on the ribs, it can cause significant distortion in the MR signal, leading to inferior quality elastograms2. In this study, we describe a new Lorentz coil shaker that introduces a separation between the Lorentz coil from the ribs shaker to avoid causing distortion, while generating high amplitude shear waves in the liver. Initial results from a phantom and liver study show that the proposed transducer leads to higher accuracy in phantom and liver elasticity estimation.Methods
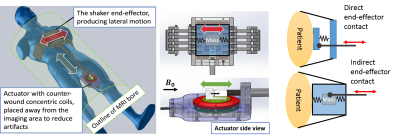
Hardware Design: The shaker uses an actively shielded electromagnetic actuator similar to our previously developed shaker for prostate imaging3. The actuator consists of two counter-wound concentric coils. One coil is the moving coil that uses the MRI magnetic field to generate the torque and the other coil is stationary and is used to cancel out the moving coil’s magnetic field and to reduce the artifacts in the image. However, the effect of the moving coil’s magnetic field cannot be cancelled fully. Especially if the actuator is located directly on top of the ROI, it can produce a significant amount of phase wrapping in the MRI phase image. Therefore, in the new shaker design, the actuator is placed away from the ROI and a mechanism is used to transfer the vibration to the end effector. At the end effector, a mass suspended by springs attached to the outer case is vibrated and the inertial forces are transferred to the end effector shell which is tied onto the patient’s chest (Fig 1). The advantage of having a moving mass and using the inertial force (indirect end effector contact) is that the end-effector can be tightly coupled to the patient’s body without applying force to the springs and reducing the vibration stroke the way this happens in direct end-effector contact.Imaging: All scans were performed on a Philips 3T Achieva scanner. The scan protocol included multi-direction multi-frequency MRE (50, 55, 60 and 65 Hz) with a multi-shot exPresso sequence3, where MRE for one direction at one frequency was scanned during a single breath-hold scan. All the scans were performed under breath-hold in the expiration position with scanning time less than 15 seconds for each scan. MRE imaging parameters included: TE= 6.9 ms, 300 x 300 mm2 FOV; 176 x 176 reconstruction matrix; 8 slices; 4 dynamics. Vibrations at different frequencies were generated using an electronic system placed outside the scanner room3; these were transmitted through a wall waveguide to the actuator inside the scanning room.
We acquired data from a CIRS 039 phantom, which has four cylindrical pots with different elasticities that correlate with the fibrosis stage of the liver. Also, MRE data were acquired from 12 healthy volunteers and 8 patients with confirmed chronic liver disease. Each subject was instructed to fast for at least 3 hours before the MRE exams.
MR Reconstruction: Measured displacements were first interpolated to an isotropic voxel size of 2mm x 2mm x 2mm. Then, a 2D bandpass filtering corresponding to an elasticity range of (1kPa- 30 kPa) was applied for displacement denoising. Finally, a local frequency estimation (LFE)4 reconstructed the elastogram for different frequencies, from which a weighted sum gives the results for fused multi-frequency elastography. For finding the mean elasticity of the liver, we have placed five 10 mm evaluation circles over different slices in regions with high amplitude waves and homogeneous elasticity. Care was taken to avoid vessels while selecting the region of interest.
Results and Discussion
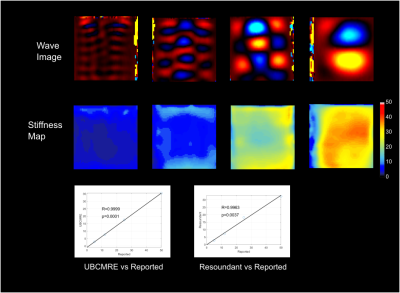
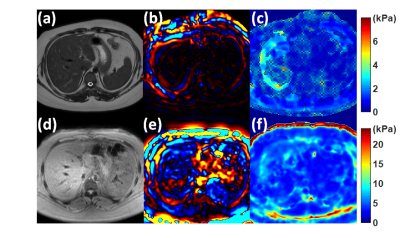
Figure 2 illustrates the results for CIRS 039 phantom for the validation of the transducer and the sequence. The reconstructed mean elasticity showed an excellent correlation with the reported elasticity of the phantom. Figure 3 shows the comparison between the Resoundant MRE (Resoundant Inc., Rochester, MN) and our system for a healthy volunteer. From the wave images, we can see the transducer provides sufficient wave coverage. The box plot in Fig.4 shows our system provides a mean elasticity of $$$4.5\pm0.16$$$ kPa for healthy volunteers (HV), and $$$6.39\pm1.79$$$ kPa for patients with liver disease (LD). Among the LD group, we found a significant difference between HV and Hepatitis C Virus (p= 0.0001) and Cirrhosis (p= 0.0001). Although the sample size is quite limited in this case, these preliminary results match with findings from previous studies on liver cirrhosis5 and hepatitis C Virus6.Conclusion
The results show that the Lorentz coil can be used to provide good wave coverage in the tissue using the magnetic field of the MRI system without introducing significant artifacts. However, the waves are attenuated inside the liver in high frequency. As the current transducer can work on an arbitrary waveform, in the future, we will investigate ways to acquire a multi-frequency vibration waveform to optimize the wave coverage with high resolution.Acknowledgements
This work was funded by NSERC, CIHR and the Charles Laszlo Chair in Biomedical Engineering held by Professor Salcudean.References
[1] Muthupillai, R., & Ehman, R. L. (1996). Magnetic resonance elastography. Nature Medicine (Vol. 2).
[2] Hirsch, S., Sack, I., & Braun, J. (2017). Magnetic resonance elastography: physical background and medical applications. John Wiley & Sons.
[3] Sahebjavaher, R. S., Frew, S., Bylinskii, A., ter Beek, L., Garteiser, P., Honarvar, M.,Salcudean, S. (2014). Prostate MR elastography with transperineal electromagnetic actuation and a fast fractionally encoded steady-state gradient echo sequence. NMR in Biomedicine, 27(7), 784–794.
[4] Manduca, A., Muthupillai, R., Rossman, P. J., Greenleaf, J. F., & Ehman, R. L. (1996). Image Processing for Magnetic Resonance Elastography. Proc. of SPIE’s Intern. Symposium Medical Imaging, 2710, 616–623.
[5] Yin, M., Talwalkar, J.A., Glaser, K.J., Manduca, A., Grimm, R.C., Rossman, P.J., Fidler, J.L. and Ehman, R.L., 2007. Assessment of hepatic fibrosis with magnetic resonance elastography. Clinical Gastroenterology and Hepatology, 5(10), pp.1207-1213.
[6] Ichikawa, S., Motosugi, U., Ichikawa, T., Sano, K., Morisaka, H., Enomoto, N., Matsuda, M., Fujii, H. and Araki, T., 2012. Magnetic resonance elastography for staging liver fibrosis in chronic hepatitis C. Magnetic Resonance in Medical Sciences, 11(4), pp.291-297.
Figures