3244
Biochemical Insights into the Molecular Mechanisms of Gadolinium Long-Term Deposition in the Human Body1Leibniz-Forschungsinstitut fuer Molekulare Pharmakologie, Berlin, Germany, 2BIOphysical Quantitative Imaging Towards Clinical Diagnosis (BIOQIC), Berlin, Germany, 3Department of Radiology, Charite Berlin, Berlin, Germany
Synopsis
The biochemical and molecular mechanisms of the gadolinium long-term deposition in biological tissues are widely unknown. In this study we prove that the observed hyperintensity of divined regions in the human body after clinical GBCA intervention can be caused by macromolecular species like glycosaminoglycans. We are able to show the importantance of sulfate groups and the influence of the sulfation state of these endogenous polysaccharides for this process by using MR relaxometry and isothermal titration calorimetry (ITC).
Purpose:
The release of Gd3+ from gadolinium-based contrast agents (GBCAs) in the presence of divalent, endogenous ions like Zn²⁺ is well known and the deposition of Gd3+ ions in the human body was repeatedly concluded as a result of hyperintensity on T1-weighted images after the administration of GBCAs [1,2]. The mechanisms leading to this long-term gadolinium deposition in the body are still unknown and represent a highly discussed topic. New insights show that the observed hyperintensities are most likely caused by Gd3+-containing macromolecular substances [3]. Endogenous sugar structures like glycosaminoglycans due to their complexing properties are candidates as competing chelators for released gadolinium ions [4]. In this study, we confirm the binding of Gd3+ ions to glycosaminoglycans and demonstrate the importance of sulfate groups for this process.Methods and materials
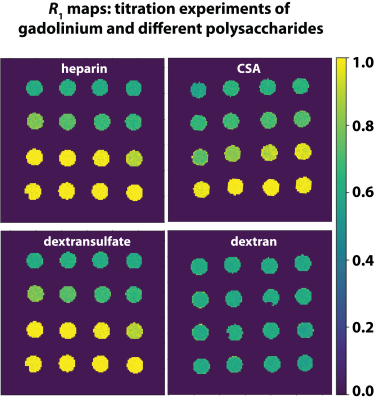
Various polysaccharides (heparin, chondroitinsulfate (CSA), dextran, and dextran sulfate) in combination with GdCl3 were used to model the chelation processes of Gd3+ ions to polysaccharides. All MR measurements were performed on a 9.4 T preclinical MRI system (Bruker, Ettlingen, Germany). T1 measurements were performed using a dephasing recovery sequence consisting of 50 π/2 pulses with subsequent gradient spoiling and image acquisition. R1 values were calculated from ROI-averaged values from R1 maps (Fig. 1).Results
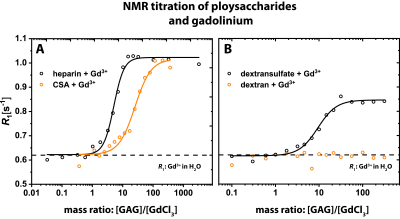
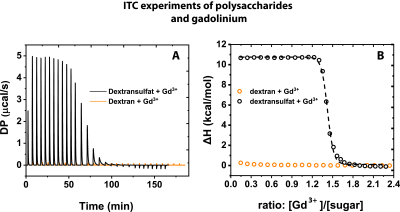
Different R1 enhancements were observed upon interaction between polysaccharides and Gd3+. Figure 2 shows R1 as a function of the mass ratio between the different polysaccharides and Gd3+ in solution. R1 increases from 0.6 s-1 (R1 of 25 μM Gd3+ in H2O) to 1.05 s-1 in both GAGs (Fig. 2A). The maxima are reached at ratios of about 10 and 100 for heparin and CSA, respectively. For dextran sulfate, R1 increases to 0.84 s-1 at a ratio of about 50, but no relevant change can be observed for dextran without sulfate groups (Fig. 2B). The lack of an R1 change indicates that no binding is happening in dextran. This is validated using isothermal titration calorimetry (ITC; Fig. 3A,B). With this technique, we could confirm a strong binding of Gd³⁺ to dextran sulfate and no binding of Gd3+ for dextran.Discussion
Glycosaminoglycans are present throughout the body and have a high chelation potential for metal ions. The fact that heparin reaches the R₁ plateau with 5 to 10 times less amount of substance than CSA can be directly linked to the different amounts of sulfate groups in these substances. Higher sulfation leads to a higher binding capacity for Gd³⁺-ions. Our results point towards an important key feature that is characteristic for ECM components. This is supported by our finding that no hyperintensity and thus no binding was observed in the absence of sulfate groups, which was validated by ITC measurements.Conclusion
Macromolecules like GAGs after binding of gadolinium have very high T1-relaxivities and can therefore be a potential explanation for the clinically observed hyperintensities long time after i.v. administration of GBCA [3]. Our results illuminate the different binding potentials of endogenous polysaccharides to bind gadolinium. The importance of the sulfation state for the binding process of released Gd-ions to polysaccharides could be proven in this study.Acknowledgements
No acknowledgement found.References
1) Kanda, T., Ishii, K., Kawaguchi, H., Kitajima, K., & Takenaka, D. (2013). High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology, 270(3), 834-841.
2) Radbruch, A., Weberling, L. D., Kieslich, P. J., Eidel, O., Burth, S., Kickingereder, P., ... & Bendszus, M. (2015). Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology, 275(3), 783-791.
3) Gianolio, E., Gregorio, E. D., & Aime, S. (2019). Chemical Insights into the Issues of Gd Retention in the Brain and Other Tissues Upon the Administration of Gd‐Containing MRI Contrast Agents. European Journal of Inorganic Chemistry, 2019(2), 137-151.
4) Taupitz, M., Stolzenburg, N., Ebert, M., Schnorr, J., Hauptmann, R., Kratz, H., ... & Wagner, S. (2013). Gadolinium‐containing magnetic resonance contrast media: investigation on the possible transchelation of Gd3+ to the glycosaminoglycan heparin. Contrast media & molecular imaging, 8(2), 108-116.
Figures