3241
Rapid and Direct Quantification of Gadolinium from Blood using Time Resolved Fluorescence
James Tranos1, Ayesha Bharadwaj Das1, Zakia Ben Youss Gironda1, Nora Butler1, Jin Zhang1, Stewart Russell2, S. Gene Kim1, and Youssef Zaim Wadghiri1
1Bernard & Irene Schwartz Center for Biomedical Imaging (CBI), Department of Radiology and Center for Advanced Imaging Innovation and Research (CAI2R), New York University School of Medicine, New York, NY, United States, 2Institute for Ultrafast Spectroscopy and Lasers, The City College of New York, New York, NY, United States
1Bernard & Irene Schwartz Center for Biomedical Imaging (CBI), Department of Radiology and Center for Advanced Imaging Innovation and Research (CAI2R), New York University School of Medicine, New York, NY, United States, 2Institute for Ultrafast Spectroscopy and Lasers, The City College of New York, New York, NY, United States
Synopsis
There are increasing needs to quantify the concentration of gadolinium for use in pharmacokinetic modeling, and traditional MR techniques prove to be inadequate. As an alternative, Time Resolved Fluorescence (TRF) can be used to directly measure the concentration of gadolinium, using a GBCA conjugated to a fluorescent antenna moiety. Here we show that TRF serves as a sensitive and direct approach to quantify the concentration of a sensitized GBCA, as a bifunctional contrast agent.
Introduction
The use of Gadolinium based contrast agents (GBCA) in clinical settings is critical for the routine screening of disease including neurological and vascular diseases as well as for the diagnosis of cancer.1 Previously, we have shown that an accurate GBCA quantification in unprocessed plasma is possible at the nanomolar level using Time Resolved Photo-induced Triplet Harvesting (TR-PTH) and energy transfer, between a sensitized gadolinium chelate donor (cs124-DTPA-Gd), and a terbium chelate acceptor.2 While this method proved very effective in quantifying GBCA within minutes, it didn’t allow for a direct assessment of Gd in blood. In the current study, we examined the innate phosphorescence of the sensitized GBCA resulting from the heavy atom effect as a sensitive method to measure the concentration of GBCA3,4 in blood without the need of additional handling. Our results demonstrate that our proposed new approach can enable direct measurement of the concentration of GBCA from blood sample via time resolved fluorescence (TRF) using a standard laboratory plate reader.Methods
Gd-DTPA Synthesis: Synthesis and purification of DTPA-cs124 was performed using the methods described by Russell et al.2Gd-DTPA-cs124 Chelation: Equimolar amounts of dry DTPA-cs124 and aqueous GdCl3 were combined and shaken at room temperature for three hours.
Spectrophotometry: TRF measurements were performed on a Biotek H1MF Plate Reader using a xenon flash lamp, and monochromators for tuning excitation and emission wavelengths. Samples were excited at 341 nm and read at 380 nm starting at 20 μs, and integrated over 1500 μs. The limit of detection (LOD) of Gd-DTPA-cs124 was determined in blood, plasma, and 1x Phosphate Buffered Saline (1x PBS) through serial dilutions of 10 mM Gd-DTPA-cs124 solution. Standard curves were established to extrapolate fluorescence measurements to specific concentrations.
Well plate MRI: A homemade 96 well plate MRI coil setup was designed based on a single loop surface coil tuned at 300Mhz Proton Larmor frequency at 7T. Relaxivity was measured using a RARE VTR sequence.
In-vivo MRI: A 3D ultrashort echo time (UTE) sequence (TR / TE = 4 / 0.028 ms) with the Golden angle Radial Spare Parallel (GRASP) method 5, namely 3D-UTE-GRASP 6, was used to demonstrate the effectiveness of our sensitized GBCA to detect brain tumors. This 3D-UTE-GRASP sequence is routinely used as part of a dynamic contrast enhanced (DCE) imaging protocol following a bolus of GBCA in saline injected through a tail vein catheter.
Results
Figure 1 illustrates the overall 96 well plate setup devised in-house in order to test and characterize our sensitized GBCA. Once filled with the variable GBCA concentrations in varying media (1x PBS, plasma or unprocessed blood), each well plate is first scanned using a plate reader in order to quantify the concentration of the sensitized GBCA (Gd-DTPA-cs124) inferred by the TRF signal. The same plate is subsequently placed on a dedicated homemade setup using a rf surface coil covering all the filled wells (shown in Figure 1.a) to be scanned using a 7-Tesla scanner in order to generate T1-maps illustrated in Figure 1.b. Figure 2 summarizes the effective relaxivity r1 of the Gd-DTPA-cs124 in various media (r1= 3.3 mM-1s-1 in 1x PBS, r1=4.8 mM-1s-1 in plasma and r1= 8.2 mM-1s-1 in unprocessed blood) by fitting the linear regression between the relaxation rate R1 (1/T1) and the corresponding concentration across the range of 0.1 µM to 3.6 mM. In comparison, the commercially available magnevist (Gd-DTPA) has an r1 relaxivity of 5.1mM-1s-1 consistent with the literature8. Figure 3 summarizes the dependence of the TRF signal with the varying concentration of the Gd-DTPA-cs124 examined within a range of 0.1µM to 10 mM. As illustrated, the TRF sensitivity decreases between 1x PBS, plasma and blood respectively. Figure 4 depicts the use of our sensitized GBCA, as an effective T1 contrast agent for detecting brain tumors. The series of images shown were obtained at 40 second intervals with the first image obtained as a baseline followed by the injection of the GBCA at a concentration of 0.1 mmol/kg.Discussion/Conclusion
In this study, our in-house well plate MRI setup enabled us to test and characterize our sensitized GBCA in a high throughput fashion concurrent to measurements performed on a plate reader. As previously demonstrated, conjugation of the cs124 moiety to Gd-DTPA resulted in an effective relaxivity for a T1 agent consistent with the literature2. In contrast to previous literature,8 r1 appears to increase in media with increasing amount of biological substrate suggesting a possible increase in affinity of our sensitized GBCA to protein present in plasma and blood. However, as can be seen in Figure 3, the sensitivity of TRF to directly quantify gadolinium in media is reduced by about 10-fold between blood and plasma and another 10-fold compared to 1x PBS. Despite this 100-fold decrease, the sensitivity in blood remains within the micromolar range which is very well suited for in-vivo small animal studies to characterize the pharmacokinetics from direct blood sampling using volume as low as 10µl. With this approach, we foresee an immediate application to help model the arterial input function in DCE studies by assessing the GBCA concentration from arterial blood sampling.Acknowledgements
This work was supported by the NSF-MRSEC Program under Award Number DMR 1420073, NSF-DMREF under Award Number DMR 1728858, the NYU Shiffrin-Myers Breast Cancer Discovery Fund, NIH/NCI R01CA160620, and the NYU CTSA grant UL1 TR000038 from the National Center for Advancing Translational Sciences, National Institutes of Health. These experiments were performed at the NYU Langone Health Preclinical Imaging Laboratory, a shared resource partially supported by the NIH/SIG 1S10OD018337-01, the Laura and Isaac Perlmutter Cancer Center Support Grant NIH/NCI 5P30CA016087, and the NIBIB Biomedical Technology Resource Center Grant NIH P41 EB017183. The authors thank Giuseppe Carlucci for facilitating access to the Radiochemistry facility and Nicholas Ramos for his assistance with the HPLC instrument.References
- 1. Kribben, A.; Witzke, O.; Hillen, U.; Barkhausen, J.; Daul, A. E.; Erbel, R. J. Am. Coll. Cardiol. 2009, 53, 1621.
- Russell S, Casey R, Hoang DM, Little BW, Olmsted PD, Rumschitzki DS, Wadghiri YZ, Fisher EA. Quantification of the plasma clearance kinetics of a gadolinium-based contrast agent by photoinduced triplet harvesting. Anal. Chem. 2012, 84, 8106−8109.
- Yersin, H. Transition Met. Rare Earth Compd. III 2004, 241, 1.
- Kleinerm, M.; Choi, S. I. J. Chem. Phys. 1968, 49, 3901.
- Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Magn Reson Med 2014;72(3):707-717.
- Zhang J, Feng L, Otazo R, Kim SG. Magn Reson Med 2019;81(1):140-152.
- NMR Biomed. 2019 Nov;32(11):e4135.
- A Y Shen, F. L. Goerner, C. Snyder, BS,† J.N. Morelli, T1 Relaxivities of Gadolinium-Based Magnetic Resonance Contrast Agents in Human Whole Blood at 1.5, 3, and 7 T. Invest Radiol 2015;50: 330–338)
- van der Weerdt AP, Klein LJ, Boellaard R, Visser CA, Visser FC, Lammertsma AA. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2001;42(11):1622-9.
- Telgmann L, Faber H, Jahn S, Melles D, Simon H, Sperling M, Karst U. Journal of chromatography A. 2012;1240:147-55.
Figures
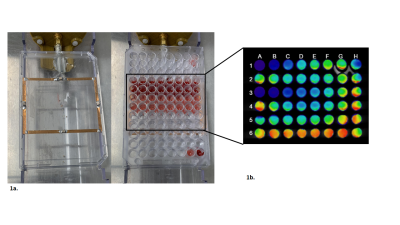
Figure 1. The overall 96 well plate setup devised in-house in order to test and characterize our sensitized GBCA. The well plate is placed over a rf surface coil (Figure 1a). The T1 maps were generated using a RARE VTR sequence (Figure 1b).
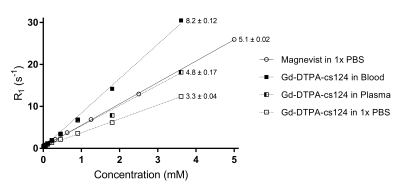
Figure 2. The effective relaxivity r1 of the Gd-DTPA-cs124 in various media
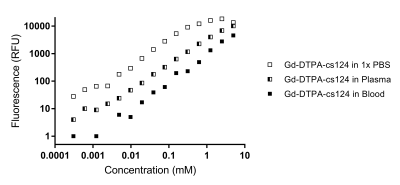
Figure 3. The dependence of the TRF signal with the varying concentrations of the Gd-DTPA-cs124.
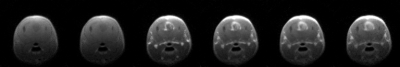
Figure 4. Use of the GBCA for an in-vivo scan of a mouse. The images were taken every 40 seconds and includes a baseline image at 0 seconds.