3220
Application of Fourier-domain Analysis Based Unwrapping Technique in Quantitative Susceptibility Mapping (QSM) of Intracerebral Hemorrhage1Department of Biomedical Engineering, University of Alberta, Edmonton, AB, Canada, 2Division of Neurology, Department of Medicine, University of Alberta, Edmonton, AB, Canada
Synopsis
QSM offers a means to measure iron content changes in hemorrhage. However, the rapid T2* decay in hemorrhage causes a severe signal loss resulting in low SNR which cause failure of standard phase unwrapping in certain cases. Fourier-domain analysis based unwrapping technique may be useful to produce susceptibility maps of hemorrhages having low SNR. This method removes residual phase wraps resulting in artifact-free QSM with boundaries of the hemorrhage area more distinct to facilitate area measurement. Thus, this method can provide more precise susceptibility maps in cases where conventional unwrapping methods fail.
Introduction
Intracerebral hemorrhage (ICH), causes approximately 15% of all stroke and leads to blood leakage into the brain parenchyma 1,2. Changes in the form or location of the released blood may be tracked using Quantitative Susceptibility Mapping (QSM). QSM studies in ICH have used multiple echo gradient echo, single echo standard Susceptibility-Weighted Imaging (SWI), or very fast sequences like single shot Echo Planar Imaging (EPI)3-5. However, particularly in acute and subacute stages, hemorrhages have a rapid T2* decay resulting in severe signal loss when using the longer echo times required in SWI and single shot EPI. The resulting low SNR often causes phase unwrapping algorithms to fail, leading to artifacts on QSM which obscure the ICH boundaries and alter the susceptibility value. In a previous paper, a post processing method, integrating image domain and Fourier-domain based analysis, was introduced to improve the accuracy of phase unwrapping for MRI images with low SNR6. QSM utilizes unwrapped phase to produce susceptibility maps and hence application of this unwrapping method may be useful to produce susceptibility maps of hemorrhages having low SNR.Purpose
To investigate the value of unwrapping phase images with Fourier-domain analysis for obtaining EPI-QSM and SWI-QSM in hemorrhage patients.Methods
Sequences: Ten patients (5male/ 5 female, age:74 ± 10 yrs.) received MRI scans at 3T with a 3D SWI sequence (TE=20ms, TR=27ms, resolution=0.85mmX0.85mmX1.5mm, acquisition time= 5 min) and a 2D single-shot gradient EPI sequence (TE=25ms, resolution=1.5mmX1.5mmX1.5mm, acquisition time=27s) described elsewhere5.Analysis: The QSM images were reconstructed using the superposed dipole inversion method for ICH-QSM4,5 with best path7 as the conventional unwrapping technique and LBV (Laplacian boundary value)8 as the background removal method.
For the Fourier-domain analysis based unwrapping method6, the complex image (size:NxxNy) was multiplied by a NxN mask to zerofill the region outside that domain. In our MRI dataset, N was optimised to be 3 and 5 for SWI and EPI respectively. The Fourier‐domain energy peak was identified, and its coordinates (in terms of distance from center of the Fourier‐ domain matrix along x and y directions) were recorded after a 2D Fourier transformation. This process was repeated for all NxN locations in the FOV to produce Fourier-space energy displacement maps (Δkx, Δky) along x and y directions. These were then converted to phase-gradient maps (Δθx and Δθy) using:
Δθx(x,y)=Δkx(x,y)*2π/Nx
Δθy(x,y)=Δky(x,y)*2π/Ny
The phase-gradient maps thus obtained were used to improve the accuracy of the phase unwrapping in the critical ROIs in hemorrhage regions where conventional unwrapping methods failed. These ROIs were visually identified from the phase images unwrapped by best path and outlined manually. The phase-gradient maps were used to calculate corrected phase values and combined using 2D numerical integration6 with boundary conditions from successfully unwrapped phase values of the conventional unwrapping method. QSM was obtained with these new unwrapped phases.
Results
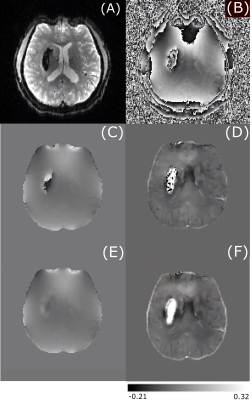
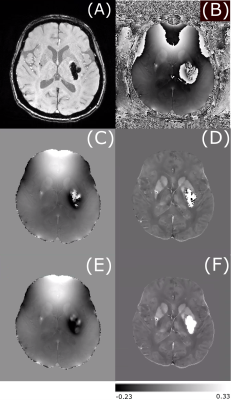
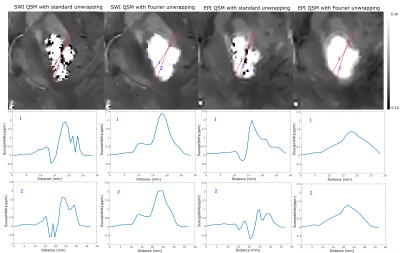
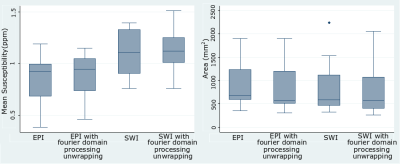
Figure 1 shows an example of the magnitude and unwrapped phase by both the conventional path-based and the Fourier-domain analysis based unwrapping methods from the EPI sequence. The residual phase wraps observed in best path unwrapping method were reduced in the Fourier-domain analysis based unwrapping methods. Figures 2 and 3 show a comparison of standard best path and Fourier-domain analysis based unwrapping methods for EPI and SWI sequences in 1 patient. Figure 4 shows a comparison of the variations in susceptibility values across the hemorrhage for QSM obtained by using both unwrapping methods for EPI and SWI sequences. It is observed that the variations are smoother for the Fourier-domain analysis based unwrapping methods. Figure 5 shows side-by-side box plot demonstrating comparison of mean susceptibility and area for 10 patients using EPI and SWI sequence for QSM with or without the addition of unwrapping using Fourier-domain analysis.Discussion
QSM requires accurate phase information and hence residual phase wraps after unwrapping can cause artifacts in susceptibility maps. In EPI due to the low resolution, phase unwrapping failure is more predominant than SWI. Using the Fourier-domain analysis based unwrapping provides QSM without such artifact, making the ICH boundaries more distinct. Hence automatic segmentation by thresholding could benefit from use of this method, despite the fact that both methods showed similar mean susceptibility and area. Even though the area measurements are similar, the median of mean susceptibility of EPI-QSM with Fourier-domain analysis-based unwrapping was higher than the conventional unwrapping method. For SWI, the patients (4 out of 10 in this patient group) with high unwrapping artifacts (as seen in example figure 3) had an increase in the mean susceptibility value. The susceptibility values of EPI-QSM are still lower than the SWI-QSM due to the lower resolution of EPI9.Conclusion
Unwrapping using Fourier-domain analysis removed residual wraps in the hemorrhage regions with low SNR. This resulted in artifact-free QSM with boundaries of the hemorrhage area more distinct to facilitate area measurement. Thus, this method can provide more precise susceptibility maps in cases where conventional unwrapping methods fail.Acknowledgements
Research support from Canadian Institutes of Health Research.
We thank Mitacs for a scholarship to AD.
References
1. Morotti A, Jessel MJ, Brouwers HB, Falcone GJ, Schwab K, Ayres AM, et al. CT angiography spot sign, hematoma expansion, and outcome in primary pontine intracerebral hemorrhage. Neurocrit Care. 2016;25:79–85.
2. Van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol, 9. 2010; 167-176.
3.Chang S, Zhang J, Liu T, Tsiouris AJ, Shou J, Nguyen T, Leifer D, Wang Y, Kovanlikaya I. Quantitative Susceptibility Mapping of Intracerebral Hemorrhages at Various Stages. J Magn Reson Imaging. 2016;44(2):420-5.
4. Sun H, Kate M, Gioia LC, Emery DJ, Butcher K, Wilman AH. Quantitative susceptibility mapping using a superposed dipole inversion method: Application to intracranial hemorrhage. Magn Reson Med. 2016; 76(3): 781-91.
5. De A, Sun H, Emery DJ, Butcher KS, Wilman AH. Rapid quantitative susceptibility mapping of intracerebral hemorrhage. J Magn Reson Imaging. 2019.
6. Chen NK, Wu PH. The use of Fourier-domain analyses for unwrapping phase images of low SNR. Magn Reson Med. 2019;82(1):356-366.
7. Abdul-Rahman HS, Gdeisat MA, Burton DR, Lalor MJ, Lilley F, Moore CJ. Fast and robust three-dimensional best path phase unwrapping algorithm. Appl. Opt. 2007; 46(26): 6623–6635.
8. Zhou D, Liu T, Spincemaille P, Wang Y. Background field removal by solving the Laplacian boundary value problem. NMR Biomed. 2014;27(3):312-9.
9. Zhou D, Cho J, Zhang J, Spincemaille P, Wang Y. Susceptibility underestimation in a high-susceptibility phantom: Dependence on imaging resolution, magnitude contrast, and other parameters. Magn Reson Med. 2017;78(3):1080-1086.
Figures