3210
Ultrashort Echo Time Quantitative Susceptibility Mapping (UTE-QSM) as a Highly Sensitive Biomarker for Hemophilic Arthropathy1University of California, San Diego, San Diego, CA, United States, 2VA San Diego Healthcare System, San Diego, CA, United States
Synopsis
Hemophilia is a genetic bleeding disorder afflicting about 20,000 people in the US and over 400,000 people in the world. Severe hemophilia is characterized by frequent joint bleeding, resulting in debilitating arthropathy because of toxic iron depositions (e.g., hemosiderin) in synovium and cartilage. Development of a sensitive, non-invasive biomarker is of high importance to determine efficacy of costly treatment plans. In this study, we investigate the feasibility of ultrashort echo time-based QSM (UTE-QSM) to identify hemosiderin deposition and to provide a sensitive biomarker for joint disease in hemophilia.
Introduction
Hemophilia is a genetic bleeding disorder that occurs in 1 out of every 5000 male births, afflicting about 20,000 people in the US alone and over 400,000 people throughout the world. Severe hemophilia is characterized by frequent joint bleeding, resulting in debilitating arthropathy because of toxic iron depositions in synovium and cartilage (hemosiderin). Frequent manifestations of iron-induced arthropathy are painful inflammatory synovial hypertrophy and osteochondral degeneration1. Subclinical joint bleeding can occur; therefore, development of a sensitive, non-invasive biomarker is of high importance to optimize efficacy of costly treatment plans and to monitor progression of disease. In magnetic resonance imaging (MRI), quantitative susceptibility mapping (QSM) is a promising technique that allows quantification of iron accumulation. However, it is challenging to measure susceptibility of highly concentrated iron with conventional MR imaging techniques due to the short T2* decay. In this study, we investigate the feasibility of ultrashort echo time-based QSM (UTE-QSM) to identify hemosiderin deposition and to provide a sensitive biomarker for joint disease in hemophilia.Methods
In this study, 3D UTE-Cones sequence was used to acquire multiple images at different TEs including UTE2 as shown in Figure 1. The multiple MR images acquired at the different TEs were input to IDEAL3 to estimate a total field map in the presence of fat signal. Then, projection onto dipole field (PDF) algorithm was applied to acquire a local field map4. The resultant local field map was input to the morphology-enabled dipole inversion (MEDI) QSM algorithm5 to estimate the final susceptibility map.To evaluate the feasibility of the proposed UTE-QSM in hemophilia, three patients with hemophilic arthropathy were recruited in accordance with the IRB. Two patients underwent knee imaging (Patient #1: 28-year-old male, Patient #2: 33-year-old male), while one patient underwent ankle imaging (Patient #3: 37-year-old male) using a 3T clinical MR system (GE-MR750). Patient #1 subsequently underwent total knee arthroplasty after the MR imaging. The tissue harvested from the surgery was immersed in saline and imaged at 3T (GE-MR750) for an additional ex vivo UTE-QSM experiment with higher spatial resolution.
In the in vivo knee and ankle experiments, the following imaging parameters were used: GE 8-channel transmit/receive knee coil; flip angle (FA)=15o; field of view (FOV)=160x160x140mm3; axial scan; matrix=160x160x100; readout bandwidth (BW)=±125kHz; TR=10ms; TE=0, 0.2, 0.4, 2.8, 3.6, and 4.4ms acquired with three dual echo scans; and total scan time=18 min. For the ex vivo experiment, the imaging parameters were matched with the knee experiment except for: homemade 30cc birdcage coil; FOV=120x120x60 mm3; matrix=240x240x120; TE=0, 0.1, 0.2, 0.3, and 0.4ms; and total scan time=57min 14sec. MEDI QSM was performed with the following parameters: radius of kernel=5, Lagrange multiplier (λ)=30000 for the in vivo and 10000 for the ex vivo experiment. QSM for the ex vivo experiment was performed without application of IDEAL.
Results
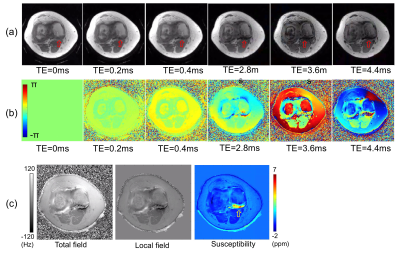
Figure 2-a and -b show magnitude and phase images at the acquired six different TEs from Patient #1, where rapid signal decay is observed in a posterior joint recess (red arrows) due to the accumulated hemosiderin. Figure 2-c shows a total field map estimated using IDEAL, a local field estimated using PDF algorithm, and the resultant susceptibility map estimated using MEDI. The estimated susceptibility map shows high susceptibility in the region where the rapid signal decay is observed (yellow arrow). Figure 3 shows the results from Patient #1, reformatted to sagittal plane. Strong signal dropoff is observed at the later TEs in the region indicated by green arrows. In the resultant susceptibility map (Figure 3-b), increased susceptibility is detected in the knee joint (yellow arrows). The estimated susceptibility in the ROI is 3.6±1.9 ppm. Figure 4 shows the results from the ex vivo experiment with the tissue harvested from the knee replacement surgery of Patient #1. The estimated susceptibility in the ROI is 4.1±2.8 ppm, which shows a similar estimate to that of the in vivo experiment. Figure 5 shows the QSM result from the other two patients (Patients #2 and #3). As shown, increased susceptibility is detected in several regions. In the ROI, the estimated susceptibility is 2.4±1.6 ppm and 1.7±0.8 ppm for Patients #2 and #3, respectively.Discussion
We have quantified and demonstrated increased intra-articular susceptibility in patients with hemophilic arthropathy, which is consistent with known hemosiderin accumulation. The hemophilic arthropathy was less progressed in Patient #2 and #3 than in Patient #1, and the susceptibility values were detected to be lower in the joints, implying feasibility of UTE-QSM as a quantitative diagnostic tool for arthropathy in hemophilia. However, further validation and optimization is required. In future works, this will be systematically verified by performing biochemical and histological analysis on the harvested tissues from hemophilic patients as a ground truth. Moreover, we will recruit a larger number of hemophilic patients to evaluate the sensitivity and specificity of the proposed biomarker based on UTE-QSM compared with other conventional imaging techniques. Combined with calibration using phantoms6, the proposed method will provide a measurement of hemosiderin concentration in hemophilia.Conclusion
We showed the feasibility of UTE-QSM in detecting hemosiderin accumulation in joints of hemophilic patients, which could provide a sensitive biomarker for toxic iron accumulation in joints to improve management of hemophilic arthropathy.Acknowledgements
The authors acknowledge grant support from NIH (R01AR075825, 2R01AR062581, 1R01 AR068987), Veterans Affairs (Merit Awards 1I01RX002604), and GE Healthcare.References
1. van Vulpen LFD, Holstein K, Martinoli C. Joint disease in haemophilia: Pathophysiology, pain and imaging. Haemophilia 2018;24:44–49
2. Lu X, Jang H, Ma Y, Jerban S, Chang E, Du J. Ultrashort Echo Time Quantitative Susceptibility Mapping (UTE-QSM) of Highly Concentrated Magnetic Nanoparticles: A Comparison Study about Different Sampling Strategies. Molecules 2019;24:1143
3. Reeder SB, Pineda AR, Wen Z, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): Application with fast spin-echo imaging. Magn. Reson. Med. 2005;54:636–644
4. Liu T, Khalidov I, de Rochefort L, et al. A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed. 2011;24:1129–1136
5. Liu J, Liu T, De Rochefort L, et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 2012;59:2560–2568
6. Jang H, Lu X, Carl M, et al. True phase quantitative susceptibility mapping using continuous single‐point imaging: a feasibility study. Magn. Reson. Med. 2019;81:1907–1914
Figures