3201
Improved Susceptibility Weighted Imaging using bipolar multi-echo acquisition and optimized processing of phase and magnitude data: CLEAR-SWI1Centre for Advanced Imaging, University of Queensland, Brisbane, Australia, 2Medical University of Vienna, Vienna, Austria, 3Medical University of Graz, Graz, Austria
Synopsis
We describe a systematic optimization of gradient-echo-based SWI at 7T, encompassing SNR in single-echo vs multi-echo acquisitions (both monopolar and bipolar), coil combination, phase unwrapping, filtering, echo weighting and correction of image inhomogeneity. The improvement achieved with the resulting Contrast-weighted, Laplace-unwrapped, bipolar multi-Echo, ASPIRE-combined, homogeneous, improved Resolution SWI (or CLEAR-SWI) is compared to ‘Standard’ single-echo, Homodyne filtered SWI in healthy subjects and patients. CLEAR-SWI reduces signal dropout, eliminates wrap artefacts and provides multi-echo phase and magnitude data for R2* mapping and QSM. Applied clinically, it provides improved visibility of multiple sclerosis lesions and the composition of tumors.
Introduction
Susceptibility Weighted Imaging (SWI [1,2]) uses high-pass filtered phase information to increase the sensitivity of gradient-echo images to frequency shifts close to susceptibility sources such as deoxygenated blood (and thereby, veins), blood products and iron. Clinically, SWI has proved valuable in imaging tumors, stroke, vascular dementia and multiple sclerosis. In common use, however, shortcomings in both the acquisition and image processing lead to artefacts and suboptimal contrast to noise ratio (CNR), particularly at ultra-high field. This study aimed to optimize all stages of the SWI process, considering SNR and CNR achieved with single-echo and multi-echo acquisitions (both monopolar and bipolar), coil combination, phase unwrapping, filtering, echo weighting and correction of image inhomogeneity. The result - Contrast-weighted, Laplace-unwrapped, bipolar multi-Echo, ASPIRE-combined, homogeneous, improved Resolution SWI (or CLEAR-SWI) - provides a dramatic improvement in image quality over conventional single-echo, Homodyne-filter-generated SWI, as well as providing multi-echo phase and magnitude data that can be used to generate R2* and Quantitative Susceptibility Maps. All the proposed steps are computationally efficient, robust and can be performed slice-wise, making them viable for online calculation on the scanner image reconstruction computer.Theory
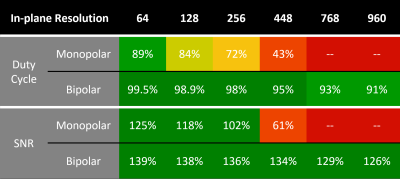
The duty cycle, or percentage of the available time that is spent sampling the echo (rather than ramping gradients and playing out refocusing gradients), was calculated for monopolar and bipolar acquisitions (parameters in Fig.1 caption). The SNR of single-echo acquisitions is given by [3] $$\mathrm{SNR}∝\frac{2T_2^*(1 - e^{-T_\mathrm{acq}/2T_2^*})e^{-T_\mathrm{E}/T_2^*}}{\sqrt{T_\mathrm{acq}}},$$ in which $$$T_2^*$$$ is the relaxation time, $$$T_\mathrm{E}$$$ the echo time and $$$T_\mathrm{acq}$$$ the sampling period. In multi-echo data in which the echoes are combined using the root-sum-of-squares (RSS), the SNR can be calculated from the SNR of the individual echoes as $$\mathrm{SNR_{ME}}=\sqrt{\sum{\mathrm{SNR}_i^2}}.$$ Duty cycle values were used for calculation of $$$T_\mathrm{acq}$$$ for the SNR calculation in Fig.1. Multi-echo SNR values are relative to the achievable SNR from a single echo acquisition and therefore exceed 100% in some cases.Methods
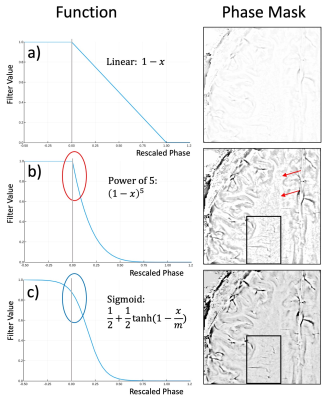
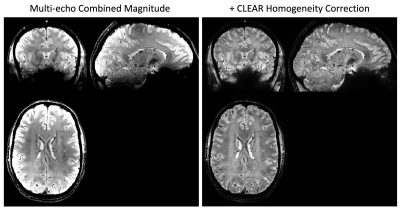
5 volunteers, 14 tumor patients and 1 multiple sclerosis patient were measured at 7T MAGNETOM with a 32-channel nova medical head coil. Informed by SNR calculations (Fig. 1), a bipolar multi-echo gradient-echo sequence was used for CLEAR-SWI, with TE=4.3:4.3:25.8ms, FA=15, BW=260Hz/px, TR=30ms, grappa=2, voxel size of 0.26x0.26x1.2mm with a total sequence time of 9min35s. For ‘Standard’ SWI, a single echo scan was acquired with identical parameters other than TE=19.3 and BW=60Hz/px. For CLEAR-SWI, phase data from the head array coil were combined using ASPIRE [4], followed by Laplacian unwrapping [5,6], combination over echoes using the magnitude as a weighting factor in CNR-optimal T2*-weighted combination [7]. A masked gaussian high-pass filter was applied in 2D with a sigma of 4 pixels and the phase mask generated using the sigmoidal function $$$f(x)=(1+\mathrm{tanh}(1-x/m))/2$$$ (Fig.2c). Magnitude data were combined over echoes using i) root-sum-of-squares (RSS) combination to optimize SNR and reduce signal dropouts and ii) CNR-weighted combination to optimize the CNR between two chosen tissue types. After homogeneity correction of the combined magnitude [8], the phase mask and magnitude were multiplied to generate the SWI. For Standard SWI, the manufacturer’s online Homodyne filtering-based reconstruction was used.Results
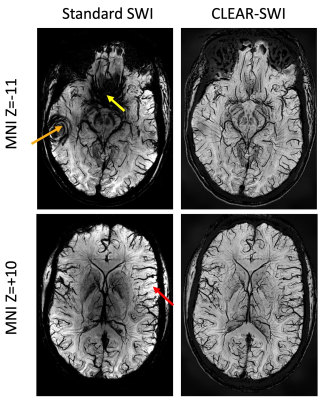
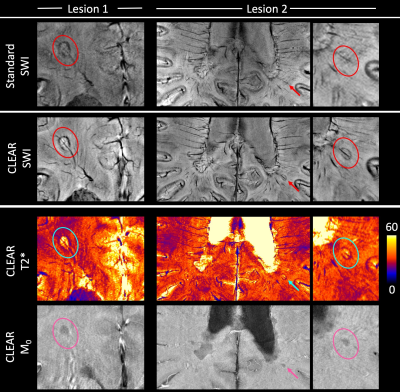
Our model of duty cycle/sampling efficiency showed advantages for bipolar acquisitions, particularly for short echo spacings and high resolution. For monopolar acquisitions and the example parameters in Fig. 1 (4 ms echo spacing, 220 mm FoV), less than 50% of the TR was spent acquiring signal above a matrix size of 448 (0.5mm resolution), with the remainder of the time being spent on fly-back gradients. For bipolar acquisitions the efficiency was, for all resolutions, above 90% and the SNR was about 30% higher than in single echo acquisitions. The sigmoid phase filter we propose reduces background noise and increases vessel contrast (Fig.2). The homogeneity filter we adopted (Fig.3) was effective in removing coil sensitivity effects. Fig. 4 illustrates the main features of CLEAR-SWI compared to Standard SWI: signal dropouts are reduced by the weighting of early echoes in those regions, phase artefacts are eliminated by the use of Laplacian unwrapping and the SWI is more homogeneous, making it easier to window and read. In imaging tumors, CLEAR-SWI showed no phase wraps or signal dropouts close to pathological tissue and the additional M0-map (the initial signal in the T2* fit, which is available due to using a multi-echo sequence) helped to identify the tumor boundaries (will be presented). In the patient with multiple sclerosis, all lesions were either more clearly visible on CLEAR-SWI than Standard SWI (e.g. Lesion 1 in Fig.5) or only visible on CLEAR-SWI (e.g. Lesion 2 in Fig.5).Conclusions
CLEAR-SWI combines optimized methods for each step of the SWI process to produce images which are dramatically improved compared to conventional, single-echo, Homodyne filtered SWI. CLEAR-SWI reduces signal dropout, increases SNR and CNR and eliminates wrap artefacts and inhomogeneities, generating images which provides additional insights into the composition of tumors and clinically relevant features in MS. In future work, CLEAR-SWI will be implemented on the image reconstructor to provide SWI for clinicians and T2* and QSMs for researchers.Acknowledgements
This study was funded by the Austrian Science Fund project FWF31452. SR was supported by the Marie Skłodowska-Curie Action MS-fMRI-QSM 794298.References
[1] Haacke, E.M., Xu, Y., Cheng, Y.-C.N., Reichenbach, J.R., 2004. Susceptibility weighted imaging (SWI). Magnetic resonance in medicine 52, 612–618.
[2] Reichenbach, J.R., Venkatesan, R., Schillinger, D.J., Kido, D.K., Haacke, E.M., 1997. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology 204, 272–277.
[3] Jutras, J.-D., Wachowicz, K., Gilbert, G., De Zanche, N., 2017. SNR efficiency of combined bipolar gradient echoes: Comparison of three-dimensional FLASH, MPRAGE, and multiparameter mapping with VFA-FLASH and MP2RAGE. Magnetic Resonance in Medicine 77, 2186–2202.
[4] Eckstein, K., Dymerska, B., Bachrata, B., Bogner, W., Poljanc, K., Trattnig, S., Robinson, S.D., 2018. Computationally Efficient Combination of Multi-channel Phase Data From Multi-echo Acquisitions (ASPIRE). Magnetic Resonance in Medicine 79, 2996–3006.
[5] Schofield, M.A., Zhu, Y., 2003. Fast phase unwrapping algorithm for interferometric applications. Opt. Lett., OL 28, 1194–1196.
[6] Rauscher, A., Sedlacik, J., Deistung, A., Mentzel, H.J., Reichenbach, J.R, 2006. Susceptibility Weighted Imaging: data acquisition, image reconstruction and clinical applications. Z Med Phys. 16(4):240-50.
[7] Wu, B., Li, W., Avram, A.V., Gho, S.-M., Liu, C., 2012. Fast and tissue-optimized mapping of magnetic susceptibility and T2* with multi-echo and multi-shot spirals. NeuroImage, Neuroergonomics: The human brain in action and at work 59, 297–305.
[8] Eckstein, K., Trattnig, S., Robinson, S.D., 2019. A Simple Homogeneity Correction for Neuroimaging at 7T, in: Proceedings of the 27th Annual Meeting ISMRM. Presented at the ISMRM, Montréal, Québec, Canada, 2011.
Figures