3155
An Efficient, Robust, and Reproducible Quantitative Radial T1 Mapping Technique
Mahesh Bharath Keerthivasan1, Marcel Dominik Nickel2, Fei Han3, Xiaodong Zhong3, Maria Altbach4, Berthold Kiefer2, and Vibhas Deshpande5
1Siemens Healthcare USA, Tucson, AZ, United States, 2Siemens Healthcare Gmbh, Erlangen, Germany, 3Siemens Healthcare USA, Los Angeles, CA, United States, 4University of Arizona, Tucson, AZ, United States, 5Siemens Healthcare USA, Austin, TX, United States
1Siemens Healthcare USA, Tucson, AZ, United States, 2Siemens Healthcare Gmbh, Erlangen, Germany, 3Siemens Healthcare USA, Los Angeles, CA, United States, 4University of Arizona, Tucson, AZ, United States, 5Siemens Healthcare USA, Austin, TX, United States
Synopsis
There has been renewed interest in the estimation of T1 relaxation times as a quantitative method for characterization of pathologies in the abdomen. While various techniques have been presented for T1 mapping they have not been systematically evaluated. In this work, we present a multi-slice radial Look-Locker FLASH technique for robust and reproducible abdominal T1 mapping. We investigate the effect of various pulse sequence and reconstruction parameters on the T1 estimation performance.
Introduction
T1-weighted imaging is routinely used for the clinical diagnosis of abdominal pathologies such as liver fibrosis and neoplasms. These are typically diagnosed based on the qualitative assessment of relative signal intensities in the liver. There has been renewed interest in the estimation of T1 relaxation times as a quantitative method for characterization of pathologies in the abdomen1-9. A variety of techniques based on variable flip angle SPGR4,9,10 and Look-Locker11,14 approaches have been proposed specifically for abdominal T1 mapping.More recently, radial inversion-recovery Look-Locker schemes have been proposed for abdominal11-13 and cardiac14,15 T1 mapping. These techniques have been shown11,14 to overcome some of the spatial resolution and scan time limitations of their Cartesian counterparts. However, the use of non-Cartesian T1 mapping techniques has not been thoroughly validated. The sensitivity of quantitative imaging techniques to pulse sequence and reconstruction parameters as well as a lack of standardized testing metrics are all important to adopt them in routine clinical practice.
In this work, we present a multi-slice radial Look-Locker FLASH technique for robust and reproducible abdominal T1 mapping. We investigate the effect of various pulse sequence and reconstruction parameters on T1 estimation performance.
Materials and Methods
Pulse Sequence and Image ReconstructionAn inversion recovery based radial Look-Locker pulse sequence with 2D FLASH readouts (radLL-FLASH) is presented in Figure1. This technique uses a single-shot acquisition strategy to continuously sample the magnetization recovery. The acquired radial spokes are grouped together to generate under-sampled k-space data which are reconstructed using a tiered view sharing approach21 to generate co-registered inversion weighted images at different inversion times (TI). T1 maps are computed by fitting the reconstructed TI images to the underlying signal model:
$$S(TI) = f(M_0,B1,T1,\alpha,TI,TR,TE) [1]$$
Various signal models have been presented in the literature for T1 estimation from inversion recovery Look-Locker schemes. While the SNAPSHOT-FLASH model by Deichmann et al16 has been commonly used, it is accurate only for low excitation flip angles. Robust fitting approaches that also estimate the excitation flip angle17 or account for slice profile variations18,19 have been proposed.
Effect of Pulse Sequence and Reconstruction Parameters
From Equation [1] we can observe that T1 estimation accuracy is sensitive to a variety of pulse sequence parameters. Thus, we evaluated the dependence of T1 estimation on the following:
- Range of flip angles and TRs
- Inversion slab thickness associated with a selective inversion preparation for multi-slice acquisitions
- Inter-slice wait-times on multi-slice acquisitions
- Temporal grouping of the radial spokes
- Extent of temporal sampling
Phantom Imaging
Data were acquired on NiCl2-doped agarose gel phantoms using the prototype radLL-FLASH sequence on a Magnetom Skyra scanner (Siemens Healthcare, Erlangen, Germany). Reference T1 values for the phantoms were obtained using an inversion-recovery single-echo spin-echo pulse sequence. The radLL-FLASH parameters were: FOV=25cm, base resolution=192, radial views=1024, TImin=30ms, slice thickness=5mm.
Results and Discussions
Effect of pulse sequence parameters:- Figure2 demonstrates the effect of excitation flip angle (Figure2A) and TR (Figure2B) on T1 estimation accuracy. While the use of FLASH readouts makes the acquired signal sensitive to B1 variations, the use of estimated FA and Block fitting models reduces variations in T1 for different flip angles.
- Since the TR controls the temporal resolution of the sampled magnetization curve and the maximum TI time sampled use of longer TRs might increase estimation errors if the null is missed.
- Bland-Altman plots in Figure3A show the need for using selective inversion slabs that are at least 1.8 times as thick as the excited slice.
- The effect of inter-slice wait times on multi-slice T1 estimation accuracy is shown in Figure3(B,C). From the plots we observe that non-selective inversion preparation is more sensitive to wait times than selective preparation for long T1s.
- The use of radial acquisitions allows flexibility in generating TI images with varying temporal resolutions. The effect of grouping radial spokes per TI is shown in Figure4A. While a lower temporal window (less radial spokes) allows for higher sampling of the recovery curve, the use of a tiered view sharing reconstruction results in more mixing of contrast.
- Figure4B shows the dependence of estimated T1 on the maximum TI value sampled. Acquiring data for more than 2.5 sec allows faithful estimation of a range of T1 species.
Inversion weighted images and T1 maps acquired on a volunteer are shown in Figure5. The use of selective inversion allows acquisition of 6 slices in a 18 sec breath-hold due to the reduced inter-slice wait times while also improving conspicuity of T1 maps due to inherent suppression of blood signal.
In summary, we have evaluated an inversion-recovery radial Look-Locker technique for robust T1 quantification.
Acknowledgements
No acknowledgement found.References
- Curcic J, Sauter M, Schwizer W, Fried M, Boesiger P, Steingoetter A. Validation of a golden angle radial sequence (GOLD) for abdominal T1 mapping during free breathing: demonstrating clinical feasibility for quantifying gastric secretion and emptying. Journal of Magnetic Resonance Imaging. 2015 Jan;41(1):157-64.
- Ding Y, Rao SX, Zhu T, Chen CZ, Li RC, Zeng MS. Liver fibrosis staging using T1 mapping on gadoxetic acid-enhanced MRI compared with DW imaging. Clinical radiology. 2015 Oct 31;70(10):1096-103.
- Treier R, Steingoetter A, Goetze O, Fox M, Fried M, Schwizer W, Boesiger P. Fast and optimized T1 mapping technique for the noninvasive quantification of gastric secretion. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008 Jul;28(1):96-102.
- Kamimura K, Fukukura Y, Yoneyama T, Takumi K, Tateyama A, Umanodan A, Shindo T, Kumagae Y, Ueno SI, Koriyama C, Nakajo M. Quantitative evaluation of liver function with T1 relaxation time index on Gd‐EOB‐DTPA‐enhanced MRI: Comparison with signal intensity‐based indices. Journal of Magnetic Resonance Imaging. 2014 Oct;40(4):884-9.
- Cassinotto C, Feldis M, Vergniol J, Mouries A, Cochet H, Lapuyade B, Hocquelet A, Juanola E, Foucher J, Laurent F, De Ledinghen V. MR relaxometry in chronic liver diseases: comparison of T1 mapping, T2 mapping, and diffusion-weighted imaging for assessing cirrhosis diagnosis and severity. European journal of radiology. 2015 Aug 1;84(8):1459-65.
- Gambarota, G., Veltien, A., Van Laarhoven, H., Philippens, M., Jonker, A., Mook, O.R., Frederiks, W.M. and Heerschap, A., 2004. Measurements of T 1 and T 2 relaxation times of colon cancer metastases in rat liver at 7 T. Magnetic Resonance Materials in Physics, Biology and Medicine, 17(3-6), pp.281-287.
- Shah, B., Anderson, S.W., Scalera, J., Jara, H. and Soto, J.A., 2011. Quantitative MR imaging: physical principles and sequence design in abdominal imaging. Radiographics, 31(3), pp.867-880.
- Chen Y, Jiang Y, Pahwa S, Ma D, Lu L, Twieg MD, Wright KL, Seiberlich N, Griswold MA, Gulani V. MR fingerprinting for rapid quantitative abdominal imaging. Radiology. 2016 Jan 21;279(1):278-86.
- Katsube T, Okada M, Kumano S, Hori M, Imaoka I, Ishii K, Kudo M, Kitagaki H, Murakami T. Estimation of liver function using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Investigative radiology. 2011 Apr 1;46(4):277-83.
- Banerjee, R., Pavlides, M., Tunnicliffe, E. M., Piechnik, S. K., Sarania, N., Philips, R., Collier, J. D., Booth, J. C., Schneider, J. E., Wang, L. M., Delaney, D. W., Fleming, K. A., Robson, M. D., Barnes, E., … Neubauer, S. (2014). Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. Journal of hepatology, 60(1), 69-77.
- Li Z, Bilgin A, Johnson K, Galons JP, Vedantham S, Martin DR, Altbach MI. Rapid High‐Resolution T1 Mapping Using a Highly Accelerated Radial Steady‐state Free‐precession Technique. Journal of Magnetic Resonance Imaging. 2018 Aug 24.
- Wang, X., Roeloffs, V., Merboldt, K. D., Voit, D., Schätz, S., & Frahm, J. (2015). Single-shot multi-slice T1 mapping at high spatial resolution–inversion-recovery FLASH with radial undersampling and iterative reconstruction. The Open Medical Imaging Journal, 9(1).
- Wang, X., Roeloffs, V., Klosowski, J., Tan, Z., Voit, D., Uecker, M., & Frahm, J. (2018). Model‐based T 1 mapping with sparsity constraints using single‐shot inversion‐recovery radial FLASH. Magnetic resonance in medicine, 79(2), 730-740.
- Wang, X., Joseph, A. A., Kalentev, O., Merboldt, K. D., Voit, D., Roeloffs, V. B., ... & Frahm, J. (2016). High-resolution myocardial T 1 mapping using single-shot inversion recovery fast low-angle shot MRI with radial undersampling and iterative reconstruction. The British journal of radiology, 89(1068), 20160255.
- Marty, B., Coppa, B., & Carlier, P. G. (2018). Fast, precise, and accurate myocardial T1 mapping using a radial MOLLI sequence with FLASH readout. Magnetic resonance in medicine, 79(3), 1387-1398.
- Deichmann, R., & Haase, A. (1992). Quantification of T1 values by SNAPSHOT-FLASH NMR imaging. Journal of Magnetic Resonance (1969), 96(3), 608-612.
- Felix Lugauer, Jens Wetzl, Christoph Forman, Manuel Schneider, Berthold Kiefer, Joachim Hornegger, Dominik Nickel, Andreas Maier, Single‑breath‑hold abdominal T1 mapping using 3D Cartesian Look‑Locker with spatiotemporal sparsity constraints, Magnetic Resonance Materials in Physics, Biology and Medicine (2018) 31:399–414
- Tran-Gia, J., Wech, T., Hahn, D., Bley, T. A., & Köstler, H. (2014). Consideration of slice profiles in inversion recovery Look–Locker relaxation parameter mapping. Magnetic resonance imaging, 32(8), 1021-1030.
- Shao, J., Rapacchi, S., Nguyen, K. L., & Hu, P. (2016). Myocardial T1 mapping at 3.0 tesla using an inversion recovery spoiled gradient echo readout and bloch equation simulation with slice profile correction (BLESSPC) T1 estimation algorithm. Journal of Magnetic Resonance Imaging, 43(2), 414-425.
- Chandarana, Hersh, Li Feng, Tobias K. Block, Andrew B. Rosenkrantz, Ruth P. Lim, James S. Babb, Daniel K. Sodickson, and Ricardo Otazo. "Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling." Investigative radiology 48, no. 1 (2013).
- Maria I. Altbach, Ali Bilgin, Zhiqiang Li, Eric W. Clarkson, Theodore P. Trouard, and Arthur F. Gmitro. Processing of Radial Fast Spin-Echo Data for Obtaining T2 Estimates from a Single K-Space Data Set. Magn. Reson. Med. 54:549-559 (2005)
Figures
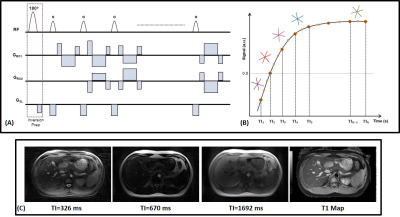
Figure
1: (A) Pulse sequence diagram and (B) sampling scheme for single shot radial
Look-Locker T1 Mapping. (C
) Representative
inversion
weighted images (3 out of 32) and the T1 map reconstructed using the prototype
radial Look-Locker sequence.
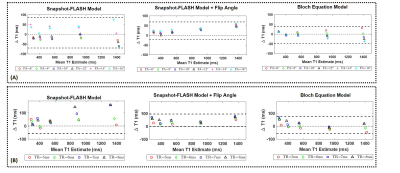
Figure
2: Effect of Pulse Sequence Parameters: Bland-Altman plots showing the effect
of (A) excitation flip angle and (B) TR on T1 estimation for three different
fitting models. The simple SNAPSHOT-FLASH model
shows large variations with flip angle, while models that estimate the
underlying B1 variations have reduced estimation error. Since the choice of TR
controls temporal resolution of the magnetization recovery curve and the
ability to faithfully sample the null point, the estimation varies based on the
T1 species considered for all three models.

Figure
3: Effect of magnetization preparation: (A) Bland-Altman plots showing the
effect of inversion slab thickness on T1 estimation for selective inversion
preparation. The effect of inter-slice wait times are shown for both (B)
selective and (C) non-selective inversion preparation. From the plots we can
observe that selective inversion preparation is less sensitive to choice of
wait time, thereby allowing improved scan efficiency.
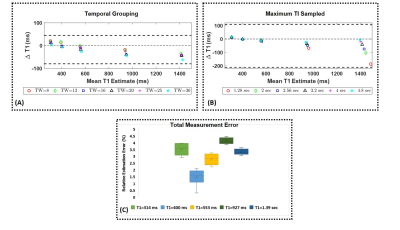
Figure
4: Effect of reconstruction parameters: (A) Bland-Altman plots showing the
dependence of estimated T1 on the number of radial spokes grouped per TI time.
Increasing the temporal window might result in signal averaging and missing the
null point. (B)
The effect of maximum TI sampled on estimation accuracy shows the needs for
longer sampling times to better estimate longer T1 species. (C ) The mean
relative T1 estimation error from 8 acquisitions spread over 3 months is
shown as a box plot for the different phantoms.
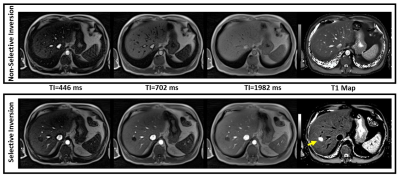
Figure
5: Inversion weighted images and T1 maps from the Radial Look-Locker FLASH
acquisition. 3 out of 32 reconstructed TI times are shown for both selective
and non-selective inversion preparation. Note that the use of a slice selective
inversion pulse allows better conspicuity of the hemangioma in the T1 map.