3140
Variable-density Fast-Spin-Echo (FSE) for volumetric inhomogeneous Magnetization Transfer (ihMT) imaging1Division of MRI research, Department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 2Global MR Applications and Workflow, GE Healthcare, Boston, MA, United States, 3CRMBM, Aix-Marseille Univ, CNRS, Marseille, France
Synopsis
While the original inhomogeneous magnetization transfer (ihMT) implementations for myelin imaging relied heavily on single-slice imaging, recent developments have enabled volumetric acquisitions using rapid gradient-echo sequences. But in vivo volumetric spin-echo acquisitions have been unexplored so far although they provide a theoretical advantage over GRE. We report here the implementation of a variable-density FSE with Compressed-Sensing for time-efficient volumetric ihMT imaging with high SNR. Provisional experiments show promising results even at high acceleration rates while also identifying areas for potential improvement, paving the way for future use of ihMT FSE for whole brain and potentially spinal cord imaging.
Introduction
Inhomogeneous Magnetization Transfer (ihMT)1 is an endogenous contrast mechanism with increased sensitivity and specificity to myelinated tissues compared to other techniques such as MT2 or diffusion imaging3, relying on the difference between a single and dual off-resonance frequency irradiation to reveal a dipolar order in membranes such as the phospholipid bilayers found in myelin4,5. While original implementations relied on single-slice sequences (e.g. EPI or single-shot-FSE) to show early applications in the healthy and diseased CNS (brain6,7 and spinal cord8,9), more recent developments allowed volumetric imaging based on rapid gradient-echo sequences (e.g. ihMT-RAGE or GRE)10,11, showing its potential for high-resolution whole brain ihMT12, but also in the cord13, paving the way for clinical studies of myelin impairment in neurological disorders. However, while being slower, a spin-echo based sequence (e.g. FSE) would be a good candidate for volumetric ihMT imaging, especially when combined with sparse-sampling strategies and Compressed-Sensing14 reconstructions to speed up acquisition while maintaining robustness. Indeed, the use of multiple refocusing flip angles has multiple benefits such as higher theoretical SNR versus GRE, possibility of long echo-train acquisitions and B0 robustness. We report here the implementation and preliminary use of a variable-density FSE sequence for volumetric ihMT imaging.Material and Methods
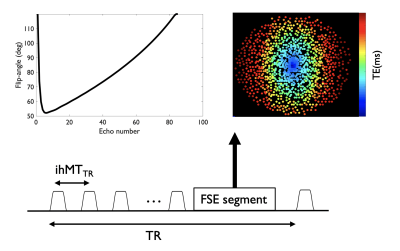
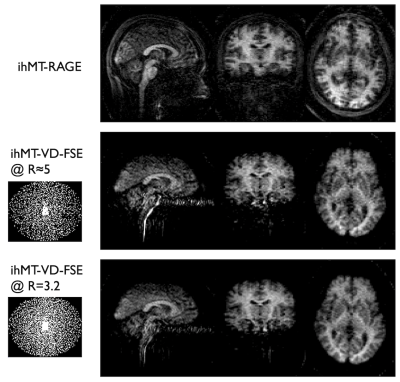
Sequence design: A MT preparation consisting of 10 off-resonance Tukey-shaped pulses (pw=5ms, ihMTTR=100ms, peak B1=15μT, f=7kHz) was implemented with a variable-density Poisson-disk FSE sequence15,16 (Fig.1). This VD-FSE consists of a 42 oversampled k-space center region acquired at each excitation followed by a 102 fully-sampled region and finally variable-density outer k-space sampling randomly distributed across excitations with a spiral-like encoding on a Cartesian grid. A variable refocusing flip-angles echo-train was used to minimize SAR and T2-blurring, with the first 4 echoes discarded because of unstable signal originating from non-CPMG components. The different MT saturations (single-positive, single-negative and dual-frequency achieved using cosine modulation) required to form the ihMT contrast (ihMT=M++M--2*Mdual) were acquired with an interleaved scheme.Experiments: A 24yo healthy male was scanned at 3T (GE Discovery MR750) using a 32-channel head coil. We acquired a sagittal VD-FSE volume (TR/TE=3500/15ms, mtx=1282, 1.8x1.8.x2.5mm3, 72 slices of 2.5mm thickness, ETL=80, echo-spacing =4.3ms) with and without the MT saturation in a total time of ≈15min. The M0 volume was acquired without variable-density sampling for accurate coil sensitivity estimation, while 3 repetitions of each saturation were acquired with an individual acceleration factor of R=8. The outer k-space sampling was randomly permuted across repetitions leading to a net acceleration of R=3.2. We compared the VD-FSE with a single-average 3D centric-out-GRE (ihMT-RAGE) ihMT acquisition (TRihMTRAGE/TR/TE=2000/4.32/1.69ms, 90 views per segment, R=2 in both phase/slice directions, Tacq=5min35s) with the same coverage and resolution.
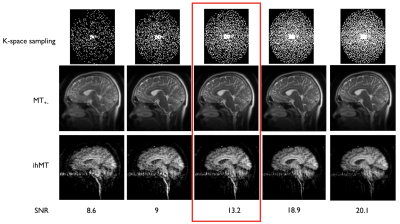
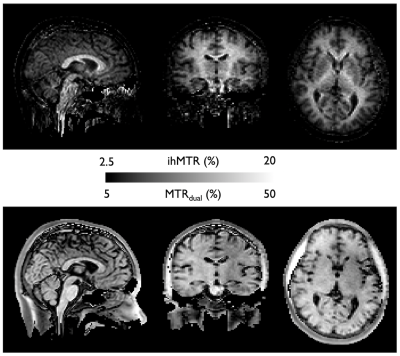
Reconstruction: Reconstruction of the VD-FSE was performed offline in MATLAB using the BART toolbox17. After coil-sensitivity estimation using ESPIRiT18 on the zero-power preparation volume, we performed a L1-wavelet Compressed-Sensing reconstruction of the complex-subtracted ihMT volume (50 iterations, λ1=0.001) and the individual MT volumes. We reconstructed the entire acquired dataset (R=3.2) but also sub-samples of the dataset (at 20/40/60/80% of the acquired samples). The 60% dataset was used for a SNR comparison with the ihMT-RAGE, as it is acquisition-time matched, by drawing a signal ROI in the corpus callosum and a noise ROI in the third ventricle (as ihMT in CSF regions is mostly noise). Finally, we calculated ihMT and MT ratios obtained from the FSE acquisition (ihMTR and MTRdual) in the corpus callosum of the volunteer.
Results
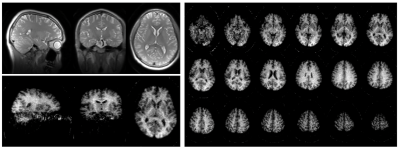
Successful reconstruction of ihMT volumes was achieved even at high acceleration rates as seen in Fig.2, although SNR decreases logically as well as reconstruction accuracy. At lower acceleration, good quality individual MT but also ihMT difference volumes could be reconstructed in less than 1 minute each. Normalized signal comparison between ihMT-RAGE and ihMT-FSE at similar Tacq seem to exhibit higher SNR using the FSE (13.2 vs 5.9). Nonetheless, we observed in the FSE some T2-decay related blurring and ghosting in the phase-encoding direction due to the use of low refocusing flip-angles (Fig.2-3). But when looking at the volume reconstructed with 100% of the acquired samples (Fig.4), one can appreciate the good white to gray matter contrast as well as overall image quality. Finally, Fig.5 shows the derived ihMT and MTdual ratios, which were measured as MTRdual=33±10% and ihMTR=15±2% in the corpus callosum, which is consistent with earlier reports.Discussion and conclusions
We have successfully implemented a variable-density FSE sequence for volumetric inhomogeneous magnetization transfer, showing its potential in a preliminary application. Although warranting thorough quantification, it provides good SNR as a spin-echo based sequence, and the k-space sampling strategy associated with CS ensures good motion robustness. Additional features such as tailored echo-trains and filtering strategies19 could improve the PSF sharpness and hence image quality of the VD-FSE by compensating for T2-decay along the echo train. While more extensive optimizations of CS weights and sampling strategy are necessary to achieve its full potential, VD-FSE does show some promise for high-SNR, high-resolution ihMT imaging of the central nervous system. Perspectives include optimizations for high-SNR spinal cord imaging combined with 4D-CS approaches to improve robustness to physiological motion, as well as assessment of the potential for high-resolution ihMT imaging similar to what has been recently proposed to study cortical myelination12.Acknowledgements
No acknowledgement found.References
1. Varma, G., Duhamel, G., Bazelaire, C. de & Alsop, D. C. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn. Reson. Med. 73, 614–622 (2015).
2. Duhamel, G. et al. Validating the sensitivity of inhomogeneous magnetization transfer (ihMT) MRI to myelin with fluorescence microscopy. NeuroImage 199, 289–303 (2019).
3. Ercan, E. et al. Microstructural correlates of 3D steady-state inhomogeneous magnetization transfer (ihMT) in the human brain white matter assessed by myelin water imaging and diffusion tensor imaging. Magn. Reson. Med. 80, 2402–2414 (2018).
4. Varma, G. et al. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. J. Magn. Reson. 260, 67–76 (2015).
5. Varma, G. et al. In vivo measurement of a new source of contrast, the dipolar relaxation time, T1D, using a modified inhomogeneous magnetization transfer (ihMT) sequence. Magn. Reson. Med. 78, 1362–1372 (2017).
6. Girard, O. M. et al. Magnetization transfer from inhomogeneously broadened lines (ihMT): Experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn. Reson. Med. 73, 2111–2121 (2015).
7. Van Obberghen, E. et al. Evaluation of the Sensitivity of Inhomogeneous Magnetization Transfer (ihMT) MRI for Multiple Sclerosis. AJNR Am. J. Neuroradiol. 39, 634–641 (2018).
8. Taso, M. et al. Tract-specific and age-related variations of the spinal cord microstructure: a multi-parametric MRI study using diffusion tensor imaging (DTI) and inhomogeneous magnetization transfer (ihMT). NMR Biomed. 29, 817–832 (2016).
9. Rasoanandrianina Henitsoa et al. Region‐specific impairment of the cervical spinal cord (SC) in amyotrophic lateral sclerosis: A preliminary study using SC templates and quantitative MRI (diffusion tensor imaging/inhomogeneous magnetization transfer). NMR Biomed. 30, e3801 (2017).
10. Varma, G. et al. 3D inhomogeneous magnetization transfer and rapid gradient echo (ihMTRAGE) imaging. in Proceedings of the Joint annual meeting of the ISMRM-ESMRMB 5503 (2018).
11. Mchinda, S. et al. Whole brain inhomogeneous magnetization transfer (ihMT) imaging: Sensitivity enhancement within a steady-state gradient echo sequence. Magn. Reson. Med. 79, 2607–2619 (2018).
12. Munsch, F. et al. Myeloarchitectonic mapping of cortical gray matter with 3D inhomogeneous magnetization transfer (ihMT). in Proceedings of the 27th annual meeting of the ISMRM 1048 (2019).
13. Troalen, T. et al. Cervical Spine inhomogeneous Magnetization Transfer (ihMT) Imaging Using ECG-Triggered 3D Rapid Acquisition Gradient-Echo (ihMT-RAGE). in Proceedings of the 27th annual meeting of the ISMRM 300 (2019).
14. Lustig, M., Donoho, D. & Pauly, J. M. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 58, 1182–1195 (2007).
15. Taso, M., Zhao, L., Guidon, A., Litwiller, D. V. & Alsop, D. C. Volumetric abdominal perfusion measurement using a pseudo-randomly sampled 3D fast-spin-echo (FSE) arterial spin labeling (ASL) sequence and compressed sensing reconstruction. Magn. Reson. Med. 82, 680–692 (2019).
16. Taso, M., Zhao, L. & Alsop, D. C. High-resolution whole brain ASL perfusion imaging using variably undersampled Cartesian Fast-Spin-Echo and Compressed Sensing reconstruction. in Proceedings of the 27th annual meeting of the ISMRM 842 (2019).
17. Uecker, M. et al. Berkeley Advanced Reconstruction Toolbox. in Proc. Intl. Soc. Mag. Reson. Med 2486 (2015).
18. Uecker, M. et al. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn. Reson. Med. 71, 990–1001 (2014).
19. Zhao, L., Chang, C.-D. & Alsop, D. C. Controlling T2 blurring in 3D RARE arterial spin labeling acquisition through optimal combination of variable flip angles and k-space filtering. Magn. Reson. Med. n/a-n/a doi:10.1002/mrm.27118.
Figures