3138
High-resolution magnetization transfer ratio maps using spiral-phyllotaxis Cartesian FLASH and compressed sensing in under five minutes
Gabriele Bonanno1,2,3, Tom Hilbert4,5,6, Arun Joseph1,2,3, Emilie Mussard4,5,6, Christoph Forman7, Gian Franco Piredda4,5,6, and Tobias Kober4,5,6
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Bern, Switzerland, 2Translational Imaging Center, sitem-insel AG, Bern, Switzerland, 3Departments of Radiology and Biomedical Research, University of Berne, Bern, Switzerland, 4Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 5Department of Radiology, University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 6LTS5, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 7Magnetic Resonance, Siemens Healthcare GmbH, Erlangen, Germany
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Bern, Switzerland, 2Translational Imaging Center, sitem-insel AG, Bern, Switzerland, 3Departments of Radiology and Biomedical Research, University of Berne, Bern, Switzerland, 4Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 5Department of Radiology, University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 6LTS5, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 7Magnetic Resonance, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Magnetization Transfer Ratio (MTR) imaging may be a valuable tool for the diagnosis and follow-up of demyelinating diseases. However, MTR maps require long scan times for whole-brain coverage and high isotropic resolution. We present a novel MTR imaging method based on a spiral-phyllotaxis Cartesian FLASH sequence and compressed sensing, called MTRSparse, and compare it to fully sampled and parallel-imaging-accelerated acquisitions in healthy volunteers. MTRSparse showed good contrast and similar MTR values in comparison to the reference method with up to 87% reduction in acquisition time. This can help facilitate implementation of MTR imaging in the clinical practice.
Introduction
In demyelinating diseases, the body’s immune system attacks the myelin sheath that surrounds the nerves’ axons causing progressive pain, and physical and cognitive dysfunction [1]. Early diagnosis of demyelination is crucial for guiding targeted treatment and reducing symptoms. The contrast mechanism of magnetization transfer (MT) originates from water bound to macromolecules such as proteins within the myelin sheath. Therefore, MT can be linked to myelin content as well as inflammation and edema [2]. In MT imaging, off-resonance pulses saturate the macromolecular proton pool (i.e. the semi-solid pool), which indirectly reduces the signal intensity of the free proton pool via magnetization transfer between these two pools [3]. A ratio of image intensities from saturated and unsaturated acquisitions (MTR) allows for a qualitative visualization of areas with MT effect. The proteins in the myelin sheath are potentially the main cause for MT effects in white matter; hence MTR might be considered an indirect measure of myelination [4]. However, long acquisition times are required to obtain three-dimensional MTR images with full-brain coverage and isotropic resolution. Therefore, these acquisitions are prone to bulk motion and their use in clinical practice is hindered. Here, we present a novel MT imaging method based on a FLASH sequence [5] and compressed sensing (called MTRSparse hereafter) and investigate its feasibility to obtain 3D MTR maps with 1-mm isotropic resolution in less than 5 min. MTRSparse was validated in healthy subjects and in comparison to fully sampled and parallel-imaging reference MTR acquisitions.Methods
IRB-approved experiments were performed in 5 healthy subjects (3 females, 29±1.6 yo) at 3 T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a commercially available 64-channel head coil. In each subject, whole-brain images were acquired with the following 3D Cartesian FLASH prototype sequences:- Reference: Fully sampled acquisition;
- GRAPPA [6]: Acquisition with a 2-fold acceleration factor in the phase encoding direction;
- MTRSparse: Acquisitions that were undersampled with a variable-density spiral-phyllotaxis pattern [7] employing an additional random perturbation of each encoding location, at different acceleration factors (Figure 1).
Reconstructions of all scans were performed inline on the scanner. Reference and GRAPPA scans used product reconstructions. For MTRSparse acquisitions, coil sensitivity maps were estimated from in-sequence calibration scans using the ESPIRiT algorithm [9] and the final image was obtained with a prototype compressed sensing algorithm as previously described [10] which used Haar wavelet regularization with 15 iterations.
Offline Analysis: MTR maps were computed in MATLAB (MathWorks, Natick, MA) as (MToff-MTon)/MToff, where MTon/off denotes scans with/without MT preparation. To account for potential subject motion, MTon images were rigidly registered [11] onto the MToff images prior to MTR calculation. Masks were obtained from MP-RAGE scans using the prototype segmentation software MorphoBox [12] in 9 bilateral VOIs (frontal, parietal, and occipital white and gray matter, corpus-callosum, thalamus, and putamen). Subsequently, the MP-RAGE images were rigidly registered [11] to each MTR image and the same transformation was applied to the masks to perform a VOI analysis in the native space of the MTR images. Median MTR values were extracted in each VOI, and the mean and standard deviation of the medians were computed across subjects. Additionally, average percentage-change of the medians with respect to reference scans was computed across subjects.
Results
All MTRSparse scans were reconstructed directly on the scanner hardware with a computation time of around two minutes per image.Qualitatively, similar image quality of MToff, MTon, and MTR images was observed when comparing MTRSparse methods to reference and GRAPPA scans. Figure 2 shows all contrasts across scans and methods acquired in an example subject. Motion artifacts can be observed in reference images that may be attributed to the lengthy acquisition time, whereas aliasing artifacts become apparent in MTRSparse images with the highest acceleration factor of 10 (Figure 2).
Figure 3 shows 3D views of another subject, where 5- and 8-fold accelerated MTRSparse compare well with conventional methods. The MTR images show similar contrast despite the reduction in acquisition time.
Mean MTR values across subjects of VOI medians were comparable across methods and the absolute mean percentage-change was lower than 2% (Figure 4). The large variation in standard deviation may be due to the limited number of subjects.
Discussion
This study suggests that 10-fold accelerated MTRSparse may be affected by aliasing artifacts, whereas 5- and 8-fold acceleration can provide similar contrast to reference methods and <2% change in MTR values, with reduced total scan times for MTR maps of 6:22 (2x3:11) min and 4:12 (2x2:06) min, respectively. For comparison, the conventional parallel-imaging protocol tested here required 17:42 (2x8:51) min total scan time. MTRSparse promises to yield whole-brain MTR maps with 1-mm isotropic resolution and adequate contrast in less than 5 min and with ~2-min inline reconstruction. This may help facilitate future use of MTR imaging in clinical practice. Further investigation in larger cohorts of healthy subjects and patients is warranted.Acknowledgements
No acknowledgement found.References
- Reich D. S., et al. Multiple Sclerosis. N Engl J Med 2018;378:169-80.
- Horsfield et al. Guidelines for Using Quantitative Magnetization Transfer Magnetic Resonance Imaging for Monitoring Treatment of Multiple Sclerosis. (2003). J. Magn. Reson. Imaging ;17:389-397
- Henkelman, R. M., Stanisz, G. J., & Graham, S. J. (2001). Magnetization transfer in MRI: a review. NMR in Biomedicine, 14(2), 57-64.
- Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol. 2004;56(3):407-415.
- Haase A, Frahm J, Matthaei D, Hänicke W, Merboldt K‐D. FLASH imaging: rapid NMR imaging using low flip angle pulses. J Magn Reson 1986; 67: 258–266.
- Griswold M, Jakob P, Heidemann R, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002; 47: 1202–1210.
- Forman, Christoph, et al. High-resolution 3D whole-heart coronary MRA: a study on the combination of data acquisition in multiple breath-holds and 1D residual respiratory motion compensation. Magn Reson Mater Phy 2014;27:435-443.
- Mugler JP, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med. 1990;15(1):152-157.
- Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med 2014;71(3):990-1001.
- Wetzl J,
Forman C, Wintersperger BJ, et al. High‐resolution
dynamic CE‐MRA of the thorax enabled by iterative TWIST reconstruction. Magn
Reson Med. 2017;77:833–840.
- S. Klein, M. Staring, K. Murphy, M.A. Viergever, J.P.W. Pluim, "elastix: a toolbox for intensity based medical image registration," IEEE Transactions on Medical Imaging (2010), vol. 29, no. 1, pp. 196 - 205.
- Schmitter, Daniel, et al. "An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer's disease." NeuroImage: Clinical 7 (2015): 7-17.
Figures
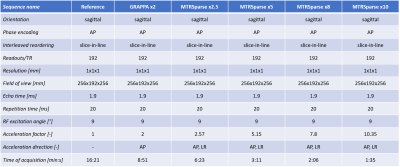
Table 1. Relevant protocol parameters of the 3D sequences
tested in this study. Each of the Reference, GRAPPA and MTRSparse sequences was
acquired twice per subject, with and without MT preparation pulse and identical
parameters as reported above. Time of acquisition is reported for one scan,
consider x2 for MTR map. For MTRSparse, the time of acquisition includes
in-sequence calibration scans for coil sensitivity maps estimation. The MT pulse
was implemented with a Gaussian pulse shape, 500° RF
excitation angle at 2 kHz off-resonance frequency.

Figure 1. Variable-density spiral-phyllotaxis pattern
in phase and slice encoding directions for undersampled 3D Cartesian trajectory
at different acceleration factors used in the MTRSparse sequence. White dots
indicate sampled lines.
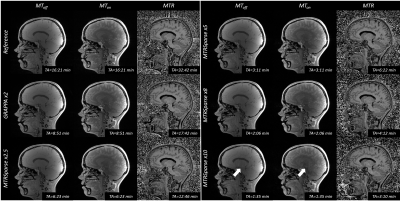
Figure 2. Sagittal
slices from all tested sequences in an example subject.
MTRSparse images compare well to Reference and GRAPPA for unsaturated (MToff)
and saturated (MTon) acquisitions as well as computed MTR maps. However,
10-fold accelerated MTRSparse images show aliasing artifacts in a sagittal view
(arrows). Note All MTon/off scans were acquired without bias field
correction, which does not affect the final MTR images due to the performed image
division.
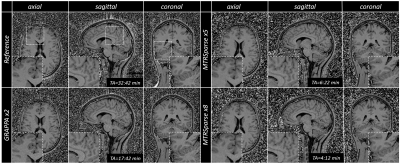
Figure 3. MTR maps from fully sampled reference, two-fold
accelerated parallel imaging (GRAPPA), and 5- and 8-fold accelerated MTRSparse acquisitions
in an example subject are shown in a 3D view. The proposed MTRSparse methods
provide good contrast and depiction of the anatomy (see insets) in comparison
to reference methods despite reduction in scan time.
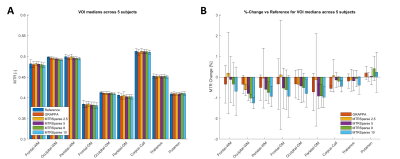
Figure 4. Quantitative results from 5 healthy subjects in
different segmented VOIs. A) Average values of medians show similar values across
techniques even for high acceleration factors. B) Average percentage change of
all methods with respect to reference scans remains lower than ±2% in all VOIs, albeit showing increased change as a
function of the acceleration factor. A, B) Error bars denote standard
deviations.