3122
Development of a standard phantom for Chemical Exchange Saturation Transfer MRI
Shu Zhang1, F. William Schuler1, Tianzhe Li1, Ken-Pin Hwang2, Mitsuharu Miyoshi3, Xinzeng Wang4, and Mark D. Pagel1
1Cancer Systems Imaging, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 2Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 3Global MR Applications & Workflow, GE Healthcare Japan, Tokyo, Japan, 4Global MR Applications & Workflow, GE Healthcare, Houston, TX, United States
1Cancer Systems Imaging, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 2Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 3Global MR Applications & Workflow, GE Healthcare Japan, Tokyo, Japan, 4Global MR Applications & Workflow, GE Healthcare, Houston, TX, United States
Synopsis
Many clinical CEST MRI methods have been developed and disseminated during the last few years, and the expansion of clinical CEST MRI will likely continue. A common phantom is needed to standardize the development and implementation of CEST methods. In this study, we have developed a clinical CEST MRI phantom with high-quality gelatin to test a range of concentrations, pH values, and T1 relaxation times. The experimental conditions of the CEST saturation and various post-saturation acquisition methods can be tested. This standard phantom can be used to support many applications within the CEST MRI research community.
Introduction
Chemical exchange saturation transfer (CEST) MRI continues to generate strong research interest for improving diagnostic assessment and treatment response monitoring.1 Although CEST MRI methods have been developed on many MRI instruments and across institutions, currently no framework exists for standardizing these methods or performing QA/QC for multicenter trials. In this study, we developed a CEST MRI phantom using high-quality gelatin with a range of concentrations, pH, and T1 relaxation times, which can be used to test a variety of CEST applications and other similar MRI experimental conditions.Methods
The main structure of the phantom was based on the clinical diffusion phantom (QalibreMD, Inc.) developed by the National Institutes of Standards and Technology (NIST) and the Quantitative Imaging Biomarkers Alliance (QIBA). It has 13 sealed vials within a water-filled, 18-cm-diameter sphere that fits within a standard head coil (Fig. 1). Three sets of phantoms were investigated: (1) 2.5, 5.0, 7.5 and 10.0% gelatin; (2) 7.5% gelatin at pH 6.0, 6.5, 7.0, 7.5 and 8.0; (3) 7.5% gelatin at pH 7.5 with T1 = 0.5, 1.0, 1.5, 2.0, 2.5, 3.0 sec (doped with Magnevist) and 3.4 sec (without Magnevist). A vial of distilled water and a vial of vegetable oil were also included.The phantoms were scanned with a 3T GE human scanner (Discovery MR750) using an 8-channel head coil at room temperature. CEST images were acquired using a 2D, single-shot fast spin echo (SSFSE) sequence with FOCUS outer volume suppression. The CEST preparation was achieved using phase cycled RF pulses.2 For each CEST scan, 29 saturation frequencies from -7 to 7 ppm were acquired, plus a reference image acquired without CEST saturation. CEST images were acquired with saturation power 0.5, 1.0, 1.5, 2.0, 3.0 μT and a fixed saturation time of 2000 ms. CEST images were also acquired with saturation time 500, 1000, 1500, 2000, 3000 ms and a fixed saturation power of 2.0 μT. WASSR field inhomogeneity correction 3 was acquired in the same scan series as CEST with a saturation power of 0.5 μT and a saturation time of 2000 ms. 11 saturation frequencies from -1.88 to 1.88 ppm were acquired for WASSR. Fat saturation pulses were used for fat suppression. Other parameters included FOV = 210 mm × 189 mm, slice thickness = 8 mm and matrix size = 128 × 128. In addition to CEST images, a B1 map was acquired using the Bloch-Siegert method 4 and a T1 map was acquired using the variable flip angle method 5.
Results
Z-spectra and MTRasym of the gelatin were obtained at different concentrations and pH (Figs. 2, 3). The peak at 3.5 ppm was not present at pH 6.0 (Fig. 2a) and was not well-defined at other low pH values (Fig. 3a), but it became visible in the Z-spectrum at pH 7.0 and increased in amplitude as pH increased (Fig. 3). The peak at 2.0 ppm was broad and visible at all pH values, and increased at higher pH values (Fig. 3). The MTRasym at 2.0 and 3.5 ppm increased as gelatin concentration increased. However, the MTRasym at both frequencies were close at 7.5 and 10.0% concentrations (Fig. 2b). The gelatin phantoms doped with Magnevist showed an increase in MTRasym as T1 increased (Fig. 4). As expected, the MTRasym at both frequencies increased as saturation power or time increased (Fig. 5).Discussion
The gelatin phantom produced CEST MRI results that met expectations with regard to the effects of concentration, pH, and T1 relaxation time. The excellent B0 and B1 homogeneity of the phantom improved the quality of these results. Reconfiguring a NIST-approved phantom provides assurance that this phantom can be established for clinical CEST MRI studies. Although recapitulating the CEST characteristics of specific tissues is very challenging, the gelatin phantom provides a reasonable approximation of endogenous CEST MRI conditions. Therefore, this study establishes a standard phantom for clinical CEST MRI. Future studies will test the incorporation of fat, glutamate, glucose, and iopamidol in the gelantin phantom, to demonstrate that the phantom can be customized for common variations of clinical CEST MRI studies. Repeatability and reproducibility tests are also on-going to establish QA/QC criteria for the clinical CEST MRI phantom. Finally, the same criteria are also being applied to develop a breast phantom, by reconfiguring the clinical breast phantom from QalibreMD for CEST MRI.Conclusion
We have established a standard phantom that can be used by the clinical CEST MRI research community to improve the validation, implementation, and dissemination of clinical CEST MRI methods.Acknowledgements
This work was supported in part by the Odyssey Program and Cockrell Foundation Award for Scientific Achievement at The University of Texas MD Anderson Cancer Center (S.Z.).References
- Jones KM, Pollard AC, Pagel MD. Clinical Applications of Chemical Exchange Saturation Transfer (CEST) MRI. J Magn Reson Imaging. 2018; 47(1): 11-27.
- Miyoshi M, Matsuda T, Kabasawa H. CEST imaging with phase cycled rectangular RF preparation pulse: analytical solution, simulation and phantom study. Proceedings of ISMRM 2014, 3299.
- Kim M, Gillen J, Landman BA, et al. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009; 61(6): 1441-1450.
- Sacolick LI, Wiesinger F, Hancu I, et al. B1 mapping by Bloch‐Siegert shift. Magn Reson Med. 2010; 63(5): 1315-1322.
- Fram EK, Herfkens RJ, Johnson GA, et al. Rapid calculation of T1 using variable flip angle gradient refocused imaging. Magn Reson Imaging. 1987; 5(3): 201-208.
Figures

Figure 1. A
phantom developed by Qalibre MD, Inc. and the National Institute for Standards
and Technolgy was modified for CEST MRI studies. The spherical phantom has the
capacity to hold 13 vials.
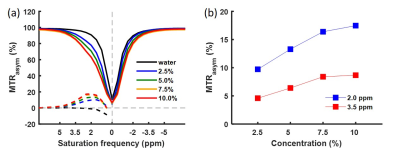
Figure 2. ROI
averaged Z-spectra and MTRasym for water and 2.5, 5.0, 7.5 and 10.0%
gelatin protein water solutions acquired with saturation time 2000 ms and
saturation power 2.0 μT (a). MTRasym at 2.0 ppm and 3.5 ppm
against gelatin concentration (b).
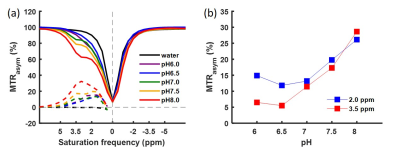
Figure 3. ROI
averaged Z-spectra and MTRasym for water and 7.5% gelatin protein
water solutions with pH 6.0, 6.5, 7.0, 7.5 and 8.0 acquired with saturation
time 2000 ms and saturation power 2.0 μT (a). MTRasym at 2.0 ppm and 3.5 ppm
against pH (b).
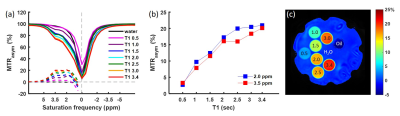
Figure 4. ROI
averaged Z-spectra and MTRasym for water and 7.5% gelatin protein
water solutions at pH 7.5 doped with Magnevist using saturation time 2000 ms
and saturation power 2.0 μT (a). MTRasym at 2.0 ppm and 3.5 ppm
against T1 (b). MTRasym map at 3.5 ppm (c).
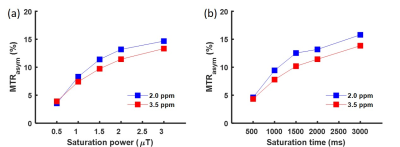
Figure 5. ROI averaged MTRasym at 2.0 ppm and 3.5
ppm against saturation power (a) and saturation time (b). The data were from
the 7.5% gelatin compartment at pH 7.5. Saturation time was maintained at 2000
ms while changing the saturation power (a). Similarly, saturation power was
maintained at 2.0 μT while changing the saturation time (b).