3118
A Steady State Approach to Positive Contrast Chemical Exchange Imaging using RACETE
Fabian Tobias Gutjahr1,2, Simon Mayer2, and Peter M Jakob2
1Comprehensive Heart Failure Center, University Hospital Wuerzburg, Wuerzburg, Germany, 2Experimental Physics 5, University Wuerzburg, Wuerzburg, Germany
1Comprehensive Heart Failure Center, University Hospital Wuerzburg, Wuerzburg, Germany, 2Experimental Physics 5, University Wuerzburg, Wuerzburg, Germany
Synopsis
RACETE is a novel method for positive contrast imaging of chemical exchange. In this work the RACETE sequence is extended to allow steady state imaging by interleaving the preparation with small flip angle RACETE read-outs. It can be shown that the efficiency of the steady state RACETE is improved over the equilibrium RACETE. This is an important step towards speeding up the acquisition process to make RACETE a viable option for future in vivo experiments.
Introduction
Chemical exchange saturation transfer (CEST) experiments serve as a sensitive measurement for substances with chemically shifted exchangeable protons1. By selectively irradiating these protons and continuous exchange to the water pool the signal in a subsequent MRI measurement is reduced. In order to visualize this negative CEST contrast a reference experiment is needed. Recently a new method utilizing a refocused acquisition of chemically exchanged excitation (RACETE) was proposed2. RACETE exploits the stimulated echo path to generate a positive contrast, eliminating the need for reference scans. The RACETE measurements previously demonstrated2 employed long repetition times TR, to allow relaxation to thermal equilibrium before each repetition, resulting in long measurement times. In this a abstract a steady-state RACETE experiment with improved efficiency is demonstrated.Methods
In Fig. 1a) the equilibrium RACETE pulse diagram is shown. First a series of preparation modules is played out on the frequency of the exchanging pool. Each excitation transfer module (ETM) encodes magnetization using the gradient Gs and stores this magnetization in the longitudinal direction. After this preparation the stimulated echo is read out using a 90°-Pulse resonant to the solvent pool. In the following delay of > 3 * T1 the magnetization relaxes to thermal equilibrium. In order to eliminate this delay, the sequence is adapted similar to a spoiled gradient echo sequence:The read-out pulse flip angle is reduced and a spoiling gradient GD is introduced to dephase any remaining transverse magnetization after each acquisition. Directly afterwards RACETE preparation is started again. This modified sequence is shown in Fig. 1 b).
In order to find the optimal number of ETMs before each read-out and the corresponding optimal readout flip angle α an analytical model using two pool Bloch-McConnell equations was derived and validated using EPG-simulations3,4 and experimental data.
All experiments were carried out on a 750 MHz MRI Scanner (Bruker BioSpin, Ettlingen, Germany) using three samples of salicylic acid (50 mmol/l) doped with Gd-DTPA to obtain different T1-values.
Results and Discussion
In Fig. 2 a) the signal in dependence on the read-out flip angle α is shown for several measurements using a different number of ETMs. In Fig. 2 b) the time-normalized signal intensities for measurements with the optimal flip angle are shown, together with a fit of the analytical model (black lines).The analytical model corresponds well to the measured and simulated data (not shown here). This model can be used to determine the optimal flip angle α, similar to the Ernst equation for FLASH imaging.
In Fig. 2b) it can be seen, that the maximum efficiency of the steady state sequence is more than two times higher for the sample with the shortest T1, compared to a measurement with TR = 10 s. The efficiency remains close to its peak value for a broad range of ETMs per read-out, simplifying parameter choice.
In Figure 3 an equilibrium (200 ETM, 1 average, TR = 11,31 s) and a steady-state RACETE image (30 ETMs, 26 averages, TR = 0,44 s) of the three samples, acquired in the same measurement time are shown, demonstrating the higher average SNR (14 to 20) in the steady state image. Furthermore the steady-state RACETE image exhibits a reduced T1-contrast, as samples with short T1 profit the most from reduced TR times.
Conclusion
Steady state RACETE can improve the efficiency of the RACETE method significantly, enabling faster acquisition times. This is an important step for future pre-clinical in vivo measurements. The analytical model matched the measured data well and can be used for parameter choice in future steady state RACETE measurements.Acknowledgements
This work was supported by grants from the Bundesministerium für Bildung und Forschung (BMBF Projekt 01EO1504)References
- Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). JMR 2000;143:79–87
- Gutjahr, F. T., Munz, E., & Jakob, P. M. (2019). Positive chemical exchange contrast in MRI using Refocused Acquisition of Chemical Exchange Transferred Excitations (RACETE). ZmedPhys, 29(2), 184–191.
- Weigel M.: Extended phase graphs: Dephasing, RF pulses, and echoes - Pure and simple. JMR (2015)
- Malik S.: Extended Phase Graph Formalism for Systems With Magnetization Transfer and Exchange, MRM 80:767-779 (2018)
Figures
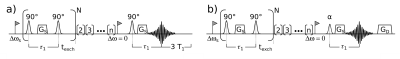
Simplified sequence diagrams of the equilibrium (a) and steady state (b) RACETE sequence. a) The number of ETM N is usually chosen to be > 200, TR = 10 ms. b) The read-out pulse flip angle is decreased and the waiting time is eliminated, however a gradient GD is introduced to dephase any remaining transverse magnetization.
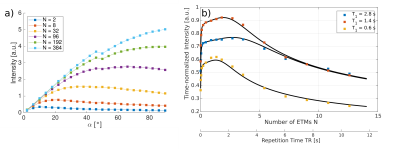
a) Intensity in dependence on the flip angle α for several numbers of ETMs per TR. b) Time normalized maximum intensity. It can be seen that the intensity is much higher for short TR measurements in comparison to TR = 10 s.
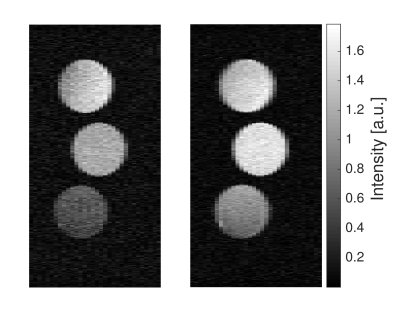
Equilibrium (left) and steady state RACETE image (right, 26 averages), acquired in the same measurement time.
The average SNR is increased by factor 1.5.