3113
Disparity between Glucose Exchange and Osmolality in Pathological Tissue using Dynamic Glucose Enhanced Magnetic Resonance Imaging (DGE-MRI)1Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 2Center for Neuroscience Imaging Research, Suwon, Republic of Korea, 3Radiology, Samsung Medical Center, Seoul, Republic of Korea
Synopsis
CEST has been used as a way to monitor glucose transport as a way of studying uptake as well as permeability. However, signal is not solely affected by exchange but also osmolality shifts with significant intravenous dosage. Using MCAO, we study disparity between these two effects and how they may lead to obfuscate signal sources. With the injection of glucose after the onset of ischemia, +1.2 ppm , -1.2 ppm, and MTR asymmetry curves behave quite differently. Both sides of the spectrum must be scrutinized to have a better picture of what is going on during glucose dosage.
Introduction
Traditionally, the administration of gadolinium based contrast agents has been utilized as a gold standard in Magnetic Resonance Imaging (MRI) for assaying alterations in permeability during pathological conditions, however, safety concerns with such agents and their effective clearance after usage have recently been rising. Alternatively, there has been growing interest in the use of the administration of glucose due to recent studies of uptake and transport of glucose through Chemical Exchange Saturation Transfer1-2 and Chemical Exchange-sensitive Spin-Lock Imaging3-4. Despite the promise of such techniques, sensitivity limitations have often led to large dosages in experimental protocol. In our previous work5, we have demonstrated that these conditions lead to overestimation in sensitivity due to T2 changes in tissue due to osmolality effects, and asymmetry analysis can circumvent the osmolality problem. That said, in normal tissue, the signal change due to exchange effects from glucose transport have been in the same direction as signal changes due to osmolality. Therefore, we wish to study the degree to which the osmolality effects of glucose transport compare to exchange effects in glucose under normal and pathological conditions. To accomplish this, we chose to study osmolality and exchange effects during uptake of intravenously administered glucose in permanent MCAO models using Dynamic Glucose Enhanced MRI.Methods
A total of 9 male C57BL/6 mice (20-30g) were anesthetized with isoflurane and controlled at 37.2±0.5°C. Prior to imaging, the middle cerebral artery was occluded through surgery. MR experiments were performed on a Bruker Biospec 9.4T/30-cm and a volume excitation and a single loop receiver coil was used for imaging. The chemical exchange-sensitive MR pulse sequence consists of a continuous wave saturation pulse (B1=1.6μT,Tirrad=3.0s) for chemical exchange contrast and a spin-echo EPI readout with a with parameters as follows: matrix size=96x48, FOV=15mmx7.5mm, slice thickness=1mm, bandwidth=300MHz, and TE/TR=10.6ms/10.0s. Background images were acquired at 300ppm and DGE images were acquired at 1.2ppm and -1.2ppm saturation images in an alternating fashion. At 2 hours after occlusion surgery, a DGE baseline of 20 minutes was acquired before glucose injection. Glucose was infused via the tail vein at a dosage of 3.5g/kg over a 3 minute period while monitoring physiological parameters for significant variations. After infusion, CEST imaging was resumed for an additional 100 minutes of acquisition.Results
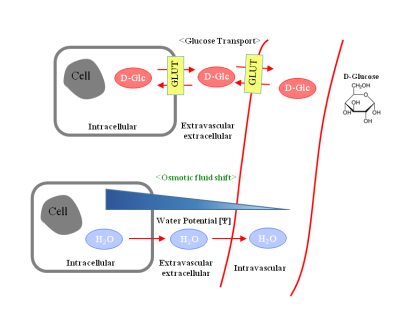
Figure 1 is a schematic of glucose transport which affects exchange induced changes in DGE signal and osmolality shifts that affect T2 induced changes in DGE signal. The top of Figure 1 shows glucose being transported into the cell which means more labile protons for exchange. The bottom shows a concentration gradient due to large soluble components in the vasculature. The osmotic shift will cause a change in tissue T2 which attenuates the signal similarly with CEST. Figure 2 shows the DGE time course in MCAO animals. Figure 2a shows the ischemic region in a representative mouse in an ADC map. Figure 2b and 2c show the DGE time course from +1.2ppm and -1.2ppm. The time course from both the healthy and ischemic tissue are similar which gives the impression that uptake is unaffected, however, there is a divergence of the two time courses at -1.2ppm at around 40 minutes. At -1.2ppm, T2 effects are prominent, however since glucose exchange is not slow there may be some influence from the exchange peak on the positive side of the spectrum. Therefore the changes may either be due to breakage in the blood brain barrier which would downplay osmolality effects or the drop in pH in the ischemic tissue which would reduce exchange rate. Exchange/leakage changes are apparently more dominant in ischemic tissue which can clearly be seen from MTR asymmetry shown in Figure 2d. On the other hand, the continuous increase of MTR asymmetry even after such a long time period suggest residual effects may remain from B0 frequency shift during acquisition.Discussion
In our previous work we compared the osmolality-induced effects on DGE signal in comparison to exchange-dependent signal changes. However, in this ischemic model we see differences in osmolality and exchange movement. Similar osmolality effects also affect T1ρ. Zu et al.6 observed these effects in their CESL study and accounted for these effects by using high power saturation to remove T2 changes from their CESL experiment. In their study, inherent T2 changes were larger in normal tissue where chemical exchange changes were larger in tumors. This may be due to osmolality and differences in the integrity of the Blood Brain Barrier between normal and tumor tissue similar to what we observe after the onset of ischemia. Only by studying both the positive and negative offset dynamics can a better idea of exchange and osmolality effect movements be understood.Conclusion
Exchange effects and osmolality effects can affect DGE or CEST signal in vastly differing manners depending on pathology so it is important to understand which effects are participating in dynamic signals and design experiments (such as deciding on measurement offsets and the necessity of asymmetry analysis) with both exchange and osmolality as considerations.Acknowledgements
This work was supported by the Institute for Basic Science (IBS-R015-D1), NIH NS100703 (USA), and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (2016R1A2A1A05004952).References
1. Xu, X., Chan, K. W., Knutsson, L., Artemov, D., Xu, J., Liu, G., ... & van Zijl, P. (2015). Dynamic glucose enhanced (DGE) MRI for combined imaging of blood–brain barrier break down and increased blood volume in brain cancer. Magnetic Resonance in Medicine, 74(6), 1556-1563.
2. Xu, X., Yadav, N. N., Knutsson, L., Hua, J., Kalyani, R., Hall, E., ... & Barker, P. (2015). Dynamic Glucose-Enhanced (DGE) MRI: Translation to Human Scanning and First Results in Glioma Patients. Tomography: a journal for imaging research, 1(2), 105.
3. Jin T, Mehrens H, Hendrich KS, Kim SG. Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. Aug 2014;34(8):1402-1410.
4. Schuenke P, Koehler C, Korzowski A, Windschuh J, Bachert P, Ladd ME, Mundiyanapurath S, Paech D, Bickelhaupt S, Bonekamp D, Schlemmer HP, Radbruch A, Zaiss M. Adiabatically prepared spin-lock approach for T1rho-based dynamic glucose enhanced MRI at ultrahigh fields. Magnetic resonance in medicine. Jul 2017;78(1):215-225.
5. Choi W, Chung JJ, Jin T, Kim SG. Chemical Effect of Osmolality on Dynamic Glucose Enhanced(DGE) MRI. Proceedings of International Society for Magnetic Resonance in Medicine; 2017; Honolulu, Hawaii. (Proceedings of International Society for Magnetic Resonance in Medicine).
6. Zu Z, Jiang X, Xu J, Gore JC. Spin-lock Imaging of 3-o-Methyl-D Glucose (3oMG) in Brain Tumors. Magnetic resonance in medicine. Sep 2018;80(3):1110-1117.
Figures