3112
Layer-dependent Transient Saturation Transfer MRI at 7T1Wolfson Brain Imaging Centre, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom, 2Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom, 3Medical Research Council Cognition and Brain Sciences Unit, University of Cambridge, Cambridge, United Kingdom
Synopsis
The human cortical mantle has distinct non-uniform layers of gray matter. In this study we used ultra high-resolution MT-weighted imaging at 7T with varying mixing times to measure the transient effects of the magnetization suppression through the primary visual cortex (V1). The MT effect was monitored across different cortical depths to evaluate the effect of the macromolecular pool (MP) and myelination.
Purpose
The human cortical mantle has distinct non-uniform layers of gray matter1,2.One of the histological features of this lamination is the arrangement of myelinated fibres3. In conventional magnetization transfer (MT) weighted MRI, radiofrequency pulses are used to saturate the longitudinal magnetization of macromolecular protons such as myelin into steady-state and the effect is measured on the water pool signal4-6. An alternative approach is to monitor the relaxation effect as a function of delay after the MT pre-saturation designated a transient MT experiment7. In this study we used ultra high-resolution MT-weighted imaging at 7T with varying mixing times to measure the transient effects of the magnetization suppression through the primary visual cortex (V1). The MT effect was monitored across different cortical depths to evaluate the effect of the macromolecular pool (MP) and myelination.Acquisition
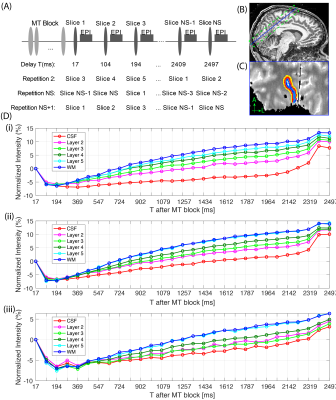
Three healthy volunteers (1 female, age: 29-36) were scanned on a 7T Siemens Terra MRI scanner. The product EPI sequence was modified (MT-EPI) to apply a train of 20 RF-pulses at 6.72ppm off-resonance, flip-angle=500º, before the EPI-readout7: 0.6mm isotropic, iPAT=3, FLASH-reference, TE/TR=22/2560ms, FoV=154mm, NS=29 slices, partial-Fourier=5/8, BW=888Hz/px, echo-spacing=1.25ms, FA=70º, 232 repetitions, 8 averages, 10 mins TA. At each repetition the slice acquisition order shifted by 2 slices such that after NS repetitions all slices were collected at all delay times (Figure 1(A)). Two repeats of the MT-EPI scan were acquired. For two subjects, a similar scan was acquired but without an MT-preparation block (116 repetitions, 4 averages). Finally, a scan was acquired where the MT block was replaced by an adiabatic inversion pulse (IR-EPI) and a sequence with identical scan parameters (except TR=4140ms, TI1=994ms, 28 slices, 112 repetitions) was collected for T1-mapping8. On all subjects the field-of-view was aligned along the calcarine cortex in V1 (Figure 1(B)).Analysis
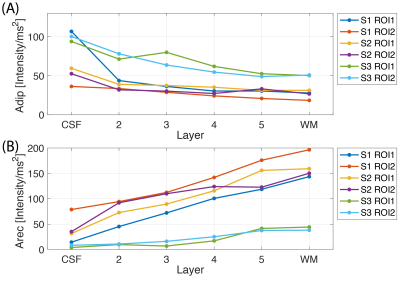
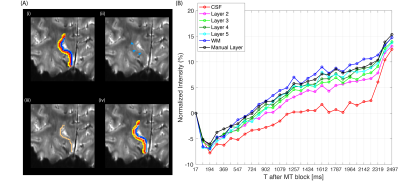
On the IR-EPI data, T1 mapping was performed with a model that considered the mono-exponential recovery of the longitudinal component of the signal based on the Bloch equations with a relaxation time T1 and was fitted to the magnitude data using a nonlinear least-squares solver8,9. For each subject, the MT-EPI data was first motion corrected with ANTs10 and then sorted and averaged. The T1 maps were registered to the MT-EPI data and the white-matter and CSF borders were drawn manually on 2 chosen regions-of-interest (ROI) in V1. On each of the ROIs, 6 cortical depths were generated11 (Figure 1(C)). The time-series MT-w signal (‘transient-MT’) was extracted from all voxels within each depth and averaged. From all subjects and ROIs, the area under the ‘transient-MT’ curves was calculated in the negative dip, ‘Adip’, and in the positive recovery, ‘Arec’. The MT-saturated image revealed a signal difference within the cortical depth in V1 consistent with the layer of Gennari. Profiles obtained from the manual segmentation of this layer were compared with the automatic method on one subject.Results
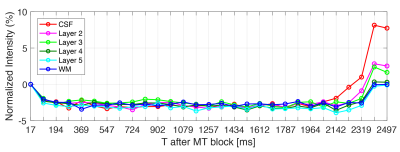
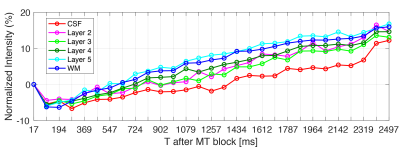
Time-varying laminar profiles from the MT saturation on cortical layers were observed in all subjects (Figure 1D). Saturation was highest in deeper layers compared to superficial layers. When MT saturation pulses were turned off, profiles showed no time dependency (Figure 2). At the tested resolution, transient-MT effects across layers were observed with one average (acquisition time approx. 1m26s) (Figure 3). However, lower SNR resulted in increased variance across the lamina data and, consequently, some overlap of the profiles. Curve areas were consistent across all subjects (Figure 4), showing an approximate 74% increase in ‘Adip’ in superficial layers compared to deeper layers. In contrast, the positive recovery area ‘Arec’ was, on average, 123% higher on deeper layers. The transient-MT profile obtained from the manually segmented layer in S2 overlapped with the generated middle layers 4 and 5 (Figure 5).Discussion and Conclusions
In this pilot experiment we show the feasibility of measuring layer-dependent saturation transfer effects on healthy young adults. Similar to 7, we analyse the saturation of macromolecular protons and the instantaneous transfer of magnetisation with the water pool. This transfer was shown to be nonuniform across depths but consistent across different regions in V1 and across subjects. Effects were observed already on a single full cycle across the EPI slice permutations with scan times as short as 1m26s, although for detecting layer effects it was necessary to increase the number of averages. The T1 mapping approach was useful to define correct CSF and white-matter boundaries as both the MT-w and T1 data shared identical EPI distortions8,9. Future work includes quantifying the macromolecular proton fraction from the multi-dimensional pool model which is formed by the cortical layers.Acknowledgements
This study was funded by the NIHR Biomedical Research Centre and the Isaac Newton Trust. CTR is funded by a Sir Henry Dale Fellowship from the Welcome Trust and the Royal Society [098436/Z/12/B]. Imaging equipment was funded by MRC Clinical Infrastructure Awards (MR/M009041/1 and MR/M008983/1).References
1. Baillarger JG. Recherches sur la structure de la couche corticale des circonvolutions du cerveau. chez J.-B. Baillière; 1840.
2. Gennari F. De peculiari structura cerebri nonnullisque ejus morbis. Ex Regio Typographeo; 1782.
3. Vogt O. Die myeloarchitektonische Felderung des menschlichen Stirnhirns. J Psychol Neurol. 1910;15(4/5):221-32.
4. Stanisz GJ, Kecojevic A, Bronskill MJ, Henkelman RM. Characterizing white matter with magnetization transfer and T2. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 1999 Dec;42(6):1128-36.
5. Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Annals of neurology. 2004 Sep;56(3):407-15.
6. Schmierer K, Wheeler‐Kingshott CA, Tozer DJ, Boulby PA, Parkes HG, Yousry TA, Scaravilli F, Barker GJ, Tofts PS, Miller DH. Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008 Feb;59(2):268-77.
7. van Gelderen P, Jiang X, Duyn JH. Rapid measurement of brain macromolecular proton fraction with transient saturation transfer MRI. Magnetic resonance in medicine. 2017 Jun;77(6):2174-85.
8. Renvall V, Witzel T, Wald LL, Polimeni JR. Automatic cortical surface reconstruction of high-resolution T1 echo planar imaging data. Neuroimage. 2016 Jul 1;134:338-54.
9. Kashyap S, Ivanov D, Havlíček M, Poser BA, Uludag K. High-resolution T1-mapping using inversion-recovery EPI and application to cortical depth-dependent fMRI at 7 Tesla. Proceedings of ISMRM, Singapore, Singapore. 2016.
10. http://stnava.github.io/ANTs/
11. https://github.com/layerfMRI/LAYNII
Figures