3110
Relaxation-compensated APT CEST imaging in breast cancer diagnostics at 7T1Radiology, German Cancer Research Center, Heidelberg, Germany, 2Medical Physics in Radiology, German Cancer Research Center, Heidelberg, Germany, 3German Cancer Research Center, Heidelberg, Germany, 4Gynecology, University Hospital Heidelberg, Heidelberg, Germany
Synopsis
Relaxation-compensated, fat-corrected and B1-corrected APT CEST MRI is a novel MR imaging technique that generates complementary information to conventional MR-mammography. In this study, the ability of this improved APT CEST metric was investigated in nine patients with newly diagnosed breast cancer. Compared to normal appearing fibroglandular breast tissue, significantly increased APT CEST signal intensities in breast cancer tissue were observed. Consequently, this MRI approach represents a contrast agent-free method that may enable a non-invasive differentiation of breast cancer and normal appearing breast tissue and, therefore, help to increase diagnostic accuracy in breast cancer imaging.
Introduction
Breast cancer is the most common tumor disease and cancer-related cause of death among women (1). In this context, Magnetic Resonance Imaging (MRI) has proven its value as a screening tool for early breast cancer detection in high-risk patients and as a diagnostic tool for preoperative tumor staging and follow-up. However, false positive findings in conventional breast MRI can lead to unnecessarily performed invasive biopsies in healthy women (2). Thus, novel MR imaging techniques are needed to complement conventional MR-mammography. In neuro-oncologic applications, relaxation-compensation and B1-correction methods have been established at 7 Tesla (7T) and have shown to yield improved diagnostic accuracy (3, 4). Recently this APT CEST metric has been extended by a novel fat correction approach and its applicability in breast tissue was demonstrated at 7T (5). In this work, we prospectively investigated the ability of this novel fat-corrected, relaxation-compensated amide proton transfer (APT) CEST MRI approach at 7T in a study cohort of nine patients with newly diagnosed breast cancer. The purpose of this study was to investigate if the approach enables a differentiation of breast cancer tissue and normal-appearing fibroglandular breast tissue.Methods
Seven female volunteers without known breast diseases and nine patients with newly diagnosed and histologically proven breast cancer were included in this prospective IRB-approved study. Relaxation-compensated APT CEST MRI of the human breast was performed at a 7 Tesla whole-body scanner (MAGNETOM 7 T, Siemens Healthineers, Erlangen, Germany) using a bilateral diagnostic breast coil (1Tx/16Rx, Rapid Biomedical GmbH, Rimpar, Germany). Pre-saturation of the custom-developed CEST sequence consisted of 297 Gaussian-shaped radiofrequency (RF) pulses (pulse length tp = 15 ms, 75 offsets, B1 = 0.6 and 0.9 µT, duty cycle (DC) = 80%, and tsat = 5.6 s) followed by a centric-reordered 2D single-slice GRE read-out (TE = 2.04 ms; TR = 3.7 ms; FoV, 196x174; matrix, 128x116; slice thickness = 5 mm; bandwidth = 1220 Hz/pixel). B1-field mapping was achieved by a modified “water shift and B1 (WASABI)” sequence (single rectangular pulse of tp=2.5ms, B1 = 7µs, followed by five 90° fat saturation pulses) (6). T1 mapping was performed using a saturation recovery preparation. APT CEST signal intensities were quantified in the tumor area and in normal appearing fibroglandular breast tissue after correction of B0/B1- field inhomogeneities, fat signal contribution, T1- and T2 –relaxation. Signal intensity differences between normal appearing breast tissue and tumor tissue were compared using the Mann–Whitney U test.Results
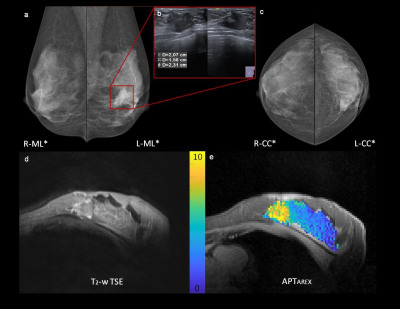
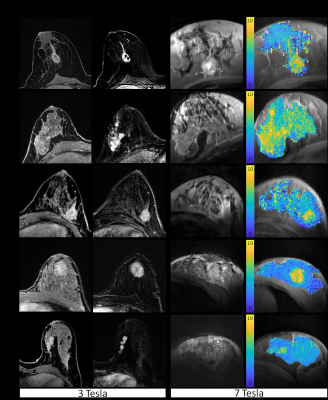
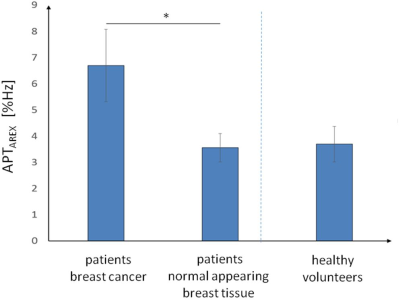
Patients showed hyperintense APTAREX contrasts in breast cancer tissue that morphologically correlated with clinical mammographic and sonographic breast examinations (Fig.1). In addition, distinct morphological correlations of increased APTAREX values in breast cancer tissue and lesion delineation at contrast-enhanced 3T clinical breast MRI were observed (Fig.2). The mean APT CEST signal intensity in breast cancer tissue (6.70 ± 1.38 %Hz) was significantly increased compared to normal appearing fibroglandular breast tissue of both, patients (3.56 ± 0.54 %Hz) and healthy volunteers (3.70 ± 0.68 %Hz) (Fig.3). Thus, the mean APT CEST signal in breast tumor tissue was increased by a factor of 1.9 compared to normal appearing breast tissue. No significant differences were found between normal appearing fibroglandular breast tissue of patients and healthy volunteers (p = 0.88) (Fig.3).Discussion
Fat-corrected, relaxation-compensated APT CEST MRI has been shown to enable detection of increased protein signal intensities in breast cancer tissue compared to normal appearing fibroglandular breast tissue. These APT CEST signal alterations in malignant breast tissue may be caused by e.g. altered protein expression (7), increased cellular proliferation (8), or protein denaturation (9, 10). In addition, this hypothesis is in line with previous studies that reported an upregulated expression level of various choline transporters and specific enzymes in breast cancer cells (11, 12).Conclusion
Fat-corrected, relaxation-compensated APT CEST MRI at 7T enabled a non-invasive differentiation of breast cancer and normal appearing fibroglandular breast tissue by quantifying increased protein-specific signal intensities in malignant breast tumors. Thus, APT CEST MRI represents a contrast agent-free method that may help to increase diagnostic accuracy in MR mammography.Acknowledgements
No acknowledgement found.References
1. Tao Z, Shi A, Lu C, et al. Breast Cancer: Epidemiology and Etiology. Cell Biochem Biophys. 2015;72(2):333-8.
2. O'Connor V, Arena E, Albright J, et al. Histological assessment of breast lesions identified exclusively by magnetic resonance. Am Surg. 2014;80(10):944-7.
3. Zaiss M, Windschuh J, Paech D, et al. Relaxation-compensated CEST-MRI of the human brain at 7T: Unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage. 2015;112:180-8.
4. Windschuh J, Zaiss M, Meissner JE, et al. Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7 T. NMR Biomed. 2015;28(5):529-37.
5. Zimmermann F, Korzowski A, Breitling J, et al. A novel normalization for amide proton transfer CEST MRI to correct for fat signal-induced artifacts: application to human breast cancer imaging. Magn Reson Med. 2019.
6. Schuenke P, Windschuh J, Roeloffs V, et al. Simultaneous mapping of water shift and B1 (WASABI)-Application to field-Inhomogeneity correction of CEST MRI data. Magn Reson Med. 2017;77(2):571-80.
7. Yan K, Fu Z, Yang C, et al. Assessing Amide Proton Transfer (APT) MRI Contrast Origins in 9 L Gliosarcoma in the Rat Brain Using Proteomic Analysis. Mol Imaging Biol. 2015;17(4):479-87.
8. Paech D, Burth S, Windschuh J, et al. Nuclear Overhauser Enhancement imaging of glioblastoma at 7 Tesla: region specific correlation with apparent diffusion coefficient and histology. PLoS One. 2015;10(3):e0121220.
9. Goerke S, Milde KS, Bukowiecki R, et al. Aggregation-induced changes in the chemical exchange saturation transfer (CEST) signals of proteins. NMR Biomed. 2017;30(1).
10. Goerke S, Zaiss M, Kunz P, et al. Signature of protein unfolding in chemical exchange saturation transfer imaging. NMR Biomed. 2015;28(7):906-13.
11. Eliyahu G, Kreizman T, Degani H. Phosphocholine as a biomarker of breast cancer: molecular and biochemical studies. Int J Cancer. 2007;120(8):1721-30.
12. Noh DY, Ahn SJ, Lee RA, et al. Overexpression of phospholipase D1 in human breast cancer tissues. Cancer Lett. 2000;161(2):207-14.
Figures