3109
Investigating the dependence of APT-CEST imaging in the human breast at 7 Tesla on the menstrual cycle1Radiology, German Cancer Research Center, Heidelberg, Germany, 2Medical Physics in Radiology, German Cancer Research Center, Heidelberg, Germany, 3Gynecology, University Hospital Heidelberg, Heidelberg, Germany, 4German Cancer Research Center, Heidelberg, Germany
Synopsis
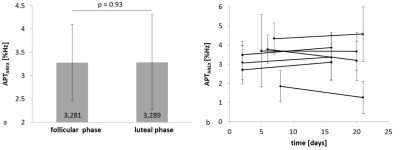
Compared to conventional dynamic contrast-enhanced MR-mammography, which is known to be significantly affected by the phase of the menstrual cycle, the menstrual cycle-related effect on APT CEST MRI in the human breast is still unknown. This is the first study investigating the influence of the menstrual cycle on APT CEST MRI in fibroglandular breast tissue of seven healthy premenopausal women. No significant signal intensity differences in fibroglandular breast tissue between the follicular and the luteal phase of the menstrual cycle were observed, which suggests that APT contrasts are comparable regardless of the phase of the menstrual cycle in premenopausal women.
Introduction
Amide proton transfer (APT)-weighted CEST MRI is a novel imaging approach that has intensively been investigated in neurooncology for tumor characterization, early therapy response assessment and prognostication as well as outcome prediction (1, 2). Furthermore, APT CEST MRI has gained attention as an additional MRI method for generating complementary information to conventional dynamic contrast-enhanced (DCE) MR-mammography in patients with newly diagnosed breast cancer (3-6). Conventional DCE MRI visualizes altered breast tissue perfusion and therefore shows dependency on the phase of the menstrual cycle (7, 8). In contrast, APT CEST MRI detects subcellular tissue characteristics due to altered protein signal intensities in breast tissue. The effect of the menstrual cycle on APT CEST imaging in the human breast is, however, still unknown. In this context, we recently applied a fat corrected, relaxation-compensated and B1-corrected APT CEST metric (9) in one healthy volunteer over the course of five weeks and observed small fluctuations of APT CEST contrast (10). In this work, we prospectively investigated the dependence between the phase of the menstrual cycle and the APT CEST contrast in healthy fibroglandular breast tissue in a cohort of seven premenopausal women. We hypothesized that the APT CEST signal intensities in breast tissue are independent of the menstrual cycle.Methods
Seven healthy volunteers (#1-7; 20-34-years-old) were examined two times during one menstrual cycle. The first examination was performed in the follicular phase (day 2-8) and the second examination in the luteal phase (day 16-21) of the menstrual cycle. Relaxation-compensated APT CEST MRI of the human breast was acquired on a 7 Tesla whole body MR scanner (MAGNETOM 7 T, Siemens Healthineers, Erlangen, Germany) using a bilateral diagnostic breast coil (1Tx/16Rx, Rapid Biomedical GmbH, Rimpar, Germany). Pre-saturation of the custom-developed CEST sequence consisted of 297 Gaussian-shaped radiofrequency (RF) pulses (pulse length tp = 15 ms, 75 offsets, B1 = 0.6 and 0.9 µT, duty cycle (DC) = 80%, and tsat = 5.6 s) followed by a centric-reordered 2D single-slice GRE read-out (TE = 2.04 ms; TR = 3.7 ms; FOV, 196x174; matrix, 128x116; slice thickness = 5 mm; bandwidth = 1220 Hz/pixel). B1-field mapping was achieved by a modified “water shift and B1 (WASABI)” sequence (single rectangular pulse of tp=2.5ms, B1 = 7µs, followed by five 90° fat saturation pulses) (11). T1 mapping was performed using a saturation recovery preparation. After correction of B0/B1- field inhomogeneities (12), fat signal contribution (9), as well as T1- and T2 –relaxation (13), semi-automated segmentation of healthy fibroglandular breast tissue was performed. Intra-individual APT CEST signal intensity differences between the follicular and luteal phase of the menstrual cycle were compared using the Wilcoxon signed-rank test.Results
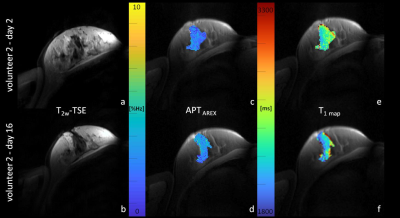
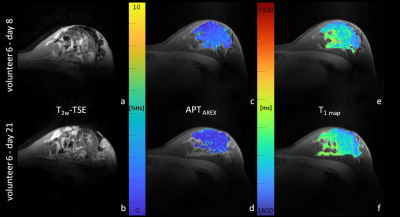
APT CEST signal intensities of fibroglandular breast tissue did not differ significantly between the follicular (3.28 ± 0.81%Hz) and the luteal phase (3.29 ± 1.02%Hz) of the menstrual cycle (p=0.93) (Fig.1a). Nevertheless, in all participants small signal intensity changes were detected (Fig.1b). Over all individuals, mean relative signal intensity change was of about -1.26% ± 16.91%. The highest intra-individual APT CEST signal intensity increase was observed in volunteer 2 with a signal intensity change of about +15% (Fig.2), the largest signal intensity decrease was about -32% in volunteer 6,(Fig.3). However, in all participants signal intensity values of the follicular and the luteal phase were within the error margins (Fig.1b). In addition, no quantitative changes of T1 relaxation times during the menstrual cycle were observed (Fig.2/3).Discussion
Numerous studies of breast tissue changes at the cellular level have reported increased proliferation, secretion activity and extracellular water volume due to altered hormonal exposures in the luteal versus follicular phase of the menstrual cycle (14, 15). These menstrual cycle related histological tissue changes are known to affect the gadolinium uptake in breast tissue during the menstrual and premenstrual phases (7, 16). In contrast, other MRI approaches and parameters in healthy fibroglandular breast tissue, e.g. diffusion-tensor-imaging and ADC (apparent diffusion coefficient), are almost constant throughout the menstrual cycle (17, 18). Similar to these parameters, this work did not reveal significant differences in the APT CEST signal intensities in breast tissue of healthy volunteers related to the phase of the menstrual cycle. In addition, no quantitative changes in T1 relaxation times were observed in this study, which is in line with previous publications (8). Thus, our results suggest that the molecular imaging approach by means of APT CEST MRI is not affected by breast tissue alterations during the menstrual cycle.Conclusion
Fat-corrected, relaxation-compensated and B1-corrected APT CEST signal intensities showed no significant alterations during the menstrual cycle, which suggests that APT CEST contrasts are not sensitive to hormonally induced changes in the breast of premenopausal women. Consequently, acquired APT CEST contrasts in premenopausal breast cancer patients are interpretable and comparable regardless of the phase of the menstrual cycle.Acknowledgements
No acknowledgement found.References
1. Paech D, Dreher C, Regnery S, Meissner JE, Goerke S, Windschuh J, et al. Relaxation-compensated amide proton transfer (APT) MRI signal intensity is associated with survival and progression in high-grade glioma patients. Eur Radiol. 2019.
2. Meissner JE, Korzowski A, Regnery S, Goerke S, Breitling J, Floca RO, et al. Early Response Assessment of Glioma Patients to Definitive Chemoradiotherapy Using Chemical Exchange Saturation Transfer Imaging at 7 T. J Magn Reson Imaging. 2019.
3. Zhang S, Seiler S, Wang X, Madhuranthakam AJ, Keupp J, Knippa EE, et al. CEST-Dixon for human breast lesion characterization at 3 T: A preliminary study. Magn Reson Med. 2018;80(3):895-903.
4. Zaric O, Farr A, Poblador Rodriguez E, Mlynarik V, Bogner W, Gruber S, et al. 7T CEST MRI: A potential imaging tool for the assessment of tumor grade and cell proliferation in breast cancer. Magn Reson Imaging. 2019;59:77-87.
5. Krikken E, Khlebnikov V, Zaiss M, Jibodh RA, van Diest PJ, Luijten PR, et al. Amide chemical exchange saturation transfer at 7 T: a possible biomarker for detecting early response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res. 2018;20(1):51.
6. Dula AN, Arlinghaus LR, Dortch RD, Dewey BE, Whisenant JG, Ayers GD, et al. Amide proton transfer imaging of the breast at 3 T: establishing reproducibility and possible feasibility assessing chemotherapy response. Magn Reson Med. 2013;70(1):216-24.
7. Kuhl CK, Bieling HB, Gieseke J, Kreft BP, Sommer T, Lutterbey G, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology. 1997;203(1):137-44.
8. Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Garrido L. Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J. 2005;11(4):236-41.
9. Zimmermann F, Korzowski A, Breitling J, Meissner JE, Schuenke P, Loi L, et al. A novel normalization for amide proton transfer CEST MRI to correct for fat signal-induced artifacts: application to human breast cancer imaging. Magn Reson Med. 2019.
10. Zimmermann F, Korzowski A, Loi L, Breitling J, Meissner JE, Zaiss M, et al. Fat corrected APT-CEST in the human breast at 7 Tesla: application to mamma carcinoma and dependency on menstrual cycle. Proceedings of the 27th ISMRM 2019, Canada, Abstract #0148. 2019.
11. Schuenke P, Windschuh J, Roeloffs V, Ladd ME, Bachert P, Zaiss M. Simultaneous mapping of water shift and B1 (WASABI)-Application to field-Inhomogeneity correction of CEST MRI data. Magn Reson Med. 2017;77(2):571-80.
12. Windschuh J, Zaiss M, Meissner JE, Paech D, Radbruch A, Ladd ME, et al. Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7 T. NMR Biomed. 2015;28(5):529-37.
13. Zaiss M, Xu J, Goerke S, Khan IS, Singer RJ, Gore JC, et al. Inverse Z-spectrum analysis for spillover-, MT-, and T1 -corrected steady-state pulsed CEST-MRI--application to pH-weighted MRI of acute stroke. NMR Biomed. 2014;27(3):240-52.
14. Vogel PM, Georgiade NG, Fetter BF, Vogel FS, McCarty KS, Jr. The correlation of histologic changes in the human breast with the menstrual cycle. Am J Pathol. 1981;104(1):23-34.
15. Potten CS, Watson RJ, Williams GT, Tickle S, Roberts SA, Harris M, et al. The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer. 1988;58(2):163-70.
16. Muller-Schimpfle M, Ohmenhauser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology. 1997;203(1):145-9.
17. Nissan N, Furman-Haran E, Shapiro-Feinberg M, Grobgeld D, Degani H. Diffusion-tensor MR imaging of the breast: hormonal regulation. Radiology. 2014;271(3):672-80.
18. Kim JY, Suh HB, Kang HJ, Shin JK, Choo KS, Nam KJ, et al. Apparent diffusion coefficient of breast cancer and normal fibroglandular tissue in diffusion-weighted imaging: the effects of menstrual cycle and menopausal status. Breast Cancer Res Treat. 2016;157(1):31-40.
Figures