3106
Differentiation between Intra-Cranial Mass Lesions using Machine Learning approach on Amide Proton Transfer-weighted (APT-w) CEST MRI1Centre for Biomedical Engineering, Indian Institute of Technology Delhi, New Delhi, India, 2Department of Radiology, 2Fortis Memorial Research Institute, Gurgaon, Haryana, India, 3Department of Biomedical Engineering, All India Institute of Medical Science, New Delhi, India
Synopsis
It is challenging to differentiate between intra-cranial mass lesions (ICMLs) due to similar appearance using conventional MRIs. Amide-proton-transfer-weighted(APT-w) MRI provides differentiation among ICMLs with lower sensitivity ad specificity. The accuracy of classification between neo-plastic mass lesions and infective mass lesions as well as differentiation between low-grade-glioma and high-grade-glioma improves by implementing machine learning classifier based on features from APT-w CEST MRI. An optimized support-vector-machine with 10-fold cross-validation and optimal set of features extracted using Random forest based feature selection provided high accuracy of around 90%.
Introduction
Classification between intra-cranial mass lesions (ICMLs) is challenging due to similar appearance and lack of enough information using conventional MRI1. Differentiation between neo-plastic and infective mass lesions as well as low-grade-glioma (LGG) and high-grade-glioma (HGG) is of utmost importance in medical diagnosis. Chemical Exchange Saturation Transfer (CEST)2 MRI is a novel contrast mechanism providing molecular information at high spatial resolution. CEST contrast from amide protons of labile proteins and peptide provides Amide-proton-transfer-weighted (APT-w)3,4 contrast which has shown promising results in different disease conditions such as tumor detection and grading4, differentiating necrosis from edema3, differentiating acute ischemic stroke from penumbra5, Alzheimer disease6, etc. Few studies7,8 showed that there was no fixed threshold in grading tumor. Recent study explored various histogram parameters apart from mean APT-w contrast to differentiate between ICMLs. Though the accuracy improves using histogram parameters for classification but still this remains challenging. Recently, artificial intelligence9 is being used to improve classification between different classes of medical data and showed promising clinical applications. Support vector machine (SVM)10 is supervised machine learning algorithm that has been widely used for classifying tumors into different grades.The purpose of the study is to evaluate the potential of artificial intelligence and implementing machine learning algorithms for better differentiation among ICMLs.Methods
Human brain APT-w MRI at 3T: A total of 32 treatment-naive patients were scanned at whole-body 3T MRI scanner (Philips). The patients include 24 neo-plastic mass-lesions (14 LGG; 10 HGG) and 8 infective mass-lesions. Conventional MRI images were acquired which included T1-w, T2-w and post contrast T1-w (PCT1-w) images. A single slice APT-w images were acquired (positioned through center of lesion) at 64 offset-frequencies (±0 ppm to ±14 ppm with step-size of 0.5 ppm, and control image at 100 ppm) using pulse sequence reported previously3. Seeded region growing algorithm followed by morphological operations were used as semi-automatic approach8 using in-house developed MATLAB_R2017 code to select region-of interest (ROI). PCT1-w images were used to segment out contrast enhancing region of lesion as described earlier8. APT-w contrast was computed as described earlier8. Different features such as mean, standard deviation (SD), median, mode, Kurtosis, skewness, entropy, full-width-half-maximum (FWHM), 10th, 25th, 50th, 75th, and 90th percentile (pr_10, pr_25, pr_50, pr_75 and pr_90), mean of the values greater than 10th, 25th, 50th, 75th, 90th percentile (mtop10, mtop25, mtop50, mtop75, mtop90), standard deviations of the values greater than 10th, 25th, 50th, 75th, 90th percentile (sdtop10, sdtop25, sdtop50, sdtop75, sdtop90) has been computed from selected ROI. A support vector machine (SVM) classifier was used for differentiating LGG from HGG as well as differentiation between neo-plastic mass lesions and infective mass lesions. The classifier was optimized w.r.t to its hyperparameter (c). A 10-fold cross-validation was performed to estimate the performance of the model on unseen or testing data. The accuracy of the SVM classifier depends on features and thus feature selection algorithm has been implemented to extract best features without loss of original information to improve accuracy. Initially, SVM was trained combining all features to compute the testing accuracy of the model. Then, optimization was carried out for feature selection or extraction using Random Forest algorithm and SVM was trained using optimal features only. Receiver-operation-characteristics (ROC) analysis was conducted to evaluate the diagnostic performance of those features which were selected from Random Forest for binary classification for improving the accuracy. Sensitivity, specificity, area-under-curve (AUC) and accuracy was computed. Confusion matrix was also generated.Results
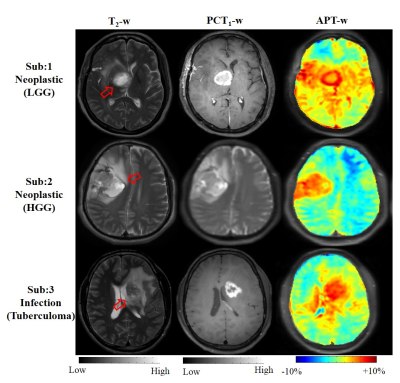
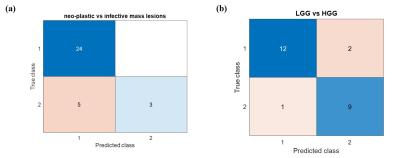
Figure-1 shows representative case of LGG, HGG and infective mass lesion and it demonstrates that similar appearance makes it difficult to differentiate. Table-1 presents the accuracy of SVM classifier using different combinations of features for classification between neo-plastic and infective mass lesions. Table-2 shows the accuracy of SVM classifier using different combinations of features for classification between LGG and HGG. Both the tables present the optimized c parameter for 10-fold classification along with AUC, sensitivity and specificity. Figure-2 shows the confusion matrix for classification between neo-plastic and infective mass lesions (Fig.2a) and LGG and HGG (Fig.2b).Discussion
Different histogram parameters of APT-w contrast provided lower accuracy, sensitivity, specificity and AUC8,11. Artificial intelligence improved the ROC analysis as presented in this study. Combination of feature sets for classification provided higher accuracy, sensitivity and specificity. The optimal features, obtained from Random Forest feature selection algorithm, supersedes the accuracy while considering the whole feature set. FWHM is a good feature or parameter for differentiation between LGG and HGG compared with conventional mean APT-w contrast. Similarly, mean of the top 75th and 90th percentiles provided to be better histogram parameters or features compared with conventional mean APT-w contrast for classifying neo-plastic from infective mass lesions. Diagnostic properties and discriminative performance were higher using machine learning classifiers.Conclusion
It is feasible to achieve high classification accuracy for differentiation between ICMLs using SVM based on optimized features obtained from APT-w MRI data. Machine learning based approach increases the specificity and sensitivity of classification method which enhances the diagnostic performance at an early stage of disease. It will assist radiologist for better clinical decision making and treatment planningAcknowledgements
This study was supported by Indian Institute of Technology Delhi and Fortis hospital, India. This study was partially supported by funding support from MATRICS, SERB-DST Grant number: MTR_2017_001021. The author thanks Virender Kumar for technical discussions.References
1. Villanueva-meyer JE, Mabray MC, Cha S. Current clinical brain tumor Imaging. NeuroSurgery 2017; 81(3): 397–415.
2. Ward KM, Aletras AH, Balaban RS. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST). J Magn Reson. 2000;143(1):79-87. doi:10.1006/jmre.1999.1956
3. Zhou J, Payen JF, Wilson DA, et al. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9:1085-1090.
4. Jones CK, Schlosser MJ, Van Zijl PCM, et al. Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med. 2006;56(3):585-592. doi:10.1002/mrm.20989
5. Tietze A, Blicher J, Mikkelsen IK, et al. Assessment of acute ischemic penumbra in patients with hyperacute stroke using amide proton trasnfer (APT) chemical exchange saturation trasnfer (CEST) MRI. NMR Biomed 2013;167-174.
6. Wang R, Li SY, Chen M, et al. Amide proton transfer magnetic resonance imaging of alzheimer’s disease at 3.0 Tesla: A preliminary study. Chin Med J (Engl). 2015;128(5):615-619.
7. Togao O, Hiwatashi A, Yamashita K, et al. Grading diffuse gliomas without intense contrast enhancement by amide proton transfer MR imaging: comparisons with diffusion- and perfusion-weighted imaging. Eur Radiol. 2017;27(2):578-588.
8. Debnath A, Gupta RK, Singh A. Evaluating the Role of Amide-Proton-Transfer Weighted contrast, Optimized for Normalization and Regions of Interest Selection, in Differentiation of Neoplastic and Infective Mass Lesions on 3T MRI. Mol Img and Biol 2019; DOI: 10.1007/s11307-019-01382-x
9. Zacharaki EI, Wang S, Chawla S, et al. Classification of brain tumor type and grade using MRI texture and shape in a machine learning scheme. Magn Reson Med 2009;62:1609–1618.
10. Cortes C, Vapnik V. Support-vector networks. Mach Learn 1995;20: 273–297
11. Debnath A, Gupta RK, Singh A. To evaluate the role of APT-w contrast, optimized for normalization and ROIs selection, in differentiating Infective and Neo-plastic Mass Lesions. International Society for Magnetic Resonance in Medicine (ISMRM): 27th Annual Meeting & Exhibition-May 2019, Canada, (Proc.Intl.Soc.Mag.Reson. 27 (2019), program number 4347)).
Figures